K2S2O8-Mediated Radical Cyclization of 1,6-Enyne for the Synthesis of Diiodonated γ-Lactams
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel and convenient K2S2O8-mediated diiodo cyclization of 1,6-enynes for the facile synthesis of functionalized γ-lactam derivatives has been developed. This reaction features mild and transition-metal-free conditions, which offer a green and efficient entry to synthetically important γ-lactam scaffolds. Mechanistic studies suggest that iodide radicals initiate the cascade cyclic transformation.
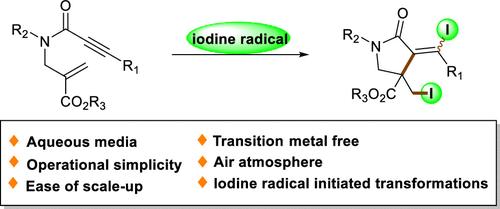
k2s2o8介导1,6-炔自由基环化合成双碘给体γ-内酰胺
研究了一种新的、方便的k2s2o8介导的1,6-炔的二碘环化反应,以方便地合成功能化γ-内酰胺衍生物。该反应具有温和且无过渡金属的特点,为合成重要的γ-内酰胺支架提供了绿色高效的入口。机理研究表明,碘化物自由基引发了级联循环转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


