Benoit Gobron, Malory Couchot, Nigel Irwin, Erick Legrand, Béatrice Bouvard, Guillaume Mabilleau
下载PDF
{"title":"Development of a First-in-Class Unimolecular Dual GIP/GLP-2 Analogue, GL-0001, for the Treatment of Bone Fragility","authors":"Benoit Gobron, Malory Couchot, Nigel Irwin, Erick Legrand, Béatrice Bouvard, Guillaume Mabilleau","doi":"10.1002/jbmr.4792","DOIUrl":null,"url":null,"abstract":"<p>Due to aging of the population, bone frailty is dramatically increasing worldwide. Although some therapeutic options exist, they do not fully protect or prevent against the occurrence of new fractures. All current drugs approved for the treatment of bone fragility target bone mass. However, bone resistance to fracture is not solely due to bone mass but relies also on bone extracellular matrix (ECM) material properties, i.e., the quality of the bone matrix component. Here, we introduce the first-in-class unimolecular dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-2 (GIP/GLP-2) analogue, GL-0001, that activates simultaneously the glucose-dependent insulinotropic polypeptide receptor (GIPr) and the glucagon-like peptide-2 receptor (GLP-2r). GL-0001 acts synergistically through a cyclic adenosine monophosphate-lysyl oxidase pathway to enhance collagen maturity. Furthermore, bilateral ovariectomy was performed in 32 BALB/c mice at 12 weeks of age prior to random allocation to either saline, dual GIP/GLP-2 analogues (GL-0001 or GL-0007) or zoledronic acid groups (<i>n</i> = 8/group). Treatment with dual GIP/GLP-2 analogues was initiated 4 weeks later for 8 weeks. At the organ level, GL-0001 modified biomechanical parameters by increasing ultimate load, postyield displacement, and energy-to-fracture of cortical bone. GL-0001 also prevented excess trabecular bone degradation at the appendicular skeleton and enhanced bone ECM material properties in cortical bone through a reduction of the mineral-to-matrix ratio and augmentation in enzymatic collagen cross-linking. These results demonstrate that targeting bone ECM material properties is a viable option to enhance bone strength and opens an innovative pathway for the treatment of patients suffering from bone fragility. © 2023 The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 5","pages":"733-748"},"PeriodicalIF":5.1000,"publicationDate":"2023-02-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4792","citationCount":"3","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4792","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 3
引用
批量引用
Abstract
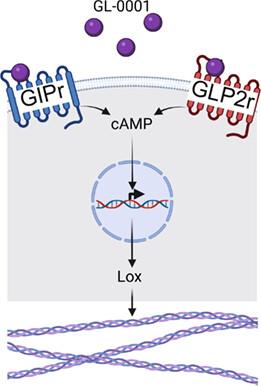
Due to aging of the population, bone frailty is dramatically increasing worldwide. Although some therapeutic options exist, they do not fully protect or prevent against the occurrence of new fractures. All current drugs approved for the treatment of bone fragility target bone mass. However, bone resistance to fracture is not solely due to bone mass but relies also on bone extracellular matrix (ECM) material properties, i.e., the quality of the bone matrix component. Here, we introduce the first-in-class unimolecular dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-2 (GIP/GLP-2) analogue, GL-0001, that activates simultaneously the glucose-dependent insulinotropic polypeptide receptor (GIPr) and the glucagon-like peptide-2 receptor (GLP-2r). GL-0001 acts synergistically through a cyclic adenosine monophosphate-lysyl oxidase pathway to enhance collagen maturity. Furthermore, bilateral ovariectomy was performed in 32 BALB/c mice at 12 weeks of age prior to random allocation to either saline, dual GIP/GLP-2 analogues (GL-0001 or GL-0007) or zoledronic acid groups (n = 8/group). Treatment with dual GIP/GLP-2 analogues was initiated 4 weeks later for 8 weeks. At the organ level, GL-0001 modified biomechanical parameters by increasing ultimate load, postyield displacement, and energy-to-fracture of cortical bone. GL-0001 also prevented excess trabecular bone degradation at the appendicular skeleton and enhanced bone ECM material properties in cortical bone through a reduction of the mineral-to-matrix ratio and augmentation in enzymatic collagen cross-linking. These results demonstrate that targeting bone ECM material properties is a viable option to enhance bone strength and opens an innovative pathway for the treatment of patients suffering from bone fragility. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
开发一流的单分子双GIP/GLP-2类似物GL-0001,用于治疗骨脆性
由于人口老龄化,骨质疏松症在世界范围内急剧增加。虽然存在一些治疗方案,但它们并不能完全保护或预防新骨折的发生。目前所有被批准用于治疗骨脆性的药物都以骨量为目标。然而,骨抗骨折性不仅仅取决于骨量,还取决于骨细胞外基质(ECM)材料的特性,即骨基质成分的质量。在这里,我们介绍了同类中第一种单分子双糖依赖性胰岛素多肽/胰高血糖素样肽-2 (GIP/GLP-2)类似物GL-0001,它可以同时激活糖依赖性胰岛素多肽受体(GIPr)和胰高血糖素样肽-2受体(GLP-2r)。GL-0001通过环腺苷单磷酸-赖氨酸氧化酶途径协同作用,提高胶原成熟度。此外,32只BALB/c小鼠在12周龄时进行双侧卵巢切除术,然后随机分配到生理盐水、双GIP/GLP-2类似物(GL-0001或GL-0007)或唑来膦酸组(n = 8/组)。4周后开始双GIP/GLP-2类似物治疗,持续8周。在器官水平上,GL-0001通过增加极限载荷、后场位移和皮质骨的骨折能量来改变生物力学参数。GL-0001还通过降低矿物质与基质的比例和增强酶促胶原交联,阻止了阑尾骨骼过度的小梁骨降解,增强了皮质骨的骨ECM材料特性。这些结果表明,靶向骨ECM材料特性是增强骨强度的可行选择,并为骨脆性患者的治疗开辟了一条创新途径。©2023作者。由Wiley期刊有限责任公司代表美国骨与矿物研究协会(ASBMR)出版的骨与矿物研究杂志。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


