Hydrogen-Atom-Transfer-Mediated Acceptorless Dehydrogenative Cross-Coupling Enabled by Multiple Catalytic Functions of Zwitterionic Triazolium Amidate
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
An unconventional cooperative catalysis for hydrogen-atom-transfer-mediated acceptorless dehydrogenative cross-coupling is described. The combined use of zwitterionic 1,2,3-triazolium amidate and an Ir-based photosensitizer as catalysts enables C–H/C–H cross-couplings between heteroatom-containing C–H donors and enamides or 1,1-diarylethenes under visible-light irradiation without the need for any oxidants, hydrogen evolution catalysts, or electrodes. A key to establishing this catalysis is the susceptibility of the conjugate acid of the triazolium amidate, amide triazolium, toward single-electron reduction to complete the catalytic cycle.
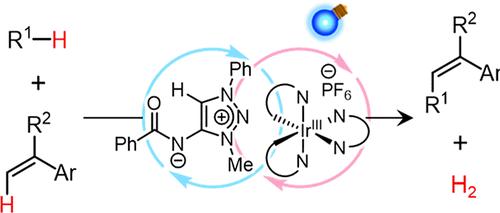
两性离子酰胺三唑的多重催化功能使氢原子转移介导的无受体脱氢交叉偶联
描述了一种非常规的协同催化氢原子转移介导的无受体脱氢交叉偶联。结合使用两性离子1,2,3-三唑酰胺和基于ir的光敏剂作为催化剂,可以在可见光照射下在含杂原子的C-H供体和酰胺或1,1-二乙烯之间实现C-H / C-H交叉偶联,而不需要任何氧化剂、析氢催化剂或电极。建立这种催化作用的关键是酰胺三唑的共轭酸(酰胺三唑)对单电子还原完成催化循环的敏感性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


