Candida albicans cell-type switching and functional plasticity in the mammalian host
IF 69.2
1区 生物学
Q1 MICROBIOLOGY
引用次数: 350
Abstract
In this Review, Noble and colleagues discuss the characteristics of the classic cell types ofCandida albicans— yeast, hyphae, pseudohyphae and chlamydospores — as well as newly identified yeast-like morphotypes, including grey and gastrointestinally induced transition (GUT) cell types, and highlight emerging knowledge about their associations with different host niches and propensities towards virulence versus commensalism. Candida albicans is a ubiquitous commensal of the mammalian microbiome and the most prevalent fungal pathogen of humans. A cell-type transition between yeast and hyphal morphologies in C. albicans was thought to underlie much of the variation in virulence observed in different host tissues. However, novel yeast-like cell morphotypes, including opaque(a/α), grey and gastrointestinally induced transition (GUT) cell types, were recently reported that exhibit marked differences in vitro and in animal models of commensalism and disease. In this Review, we explore the characteristics of the classic cell types — yeast, hyphae, pseudohyphae and chlamydospores — as well as the newly identified yeast-like morphotypes. We highlight emerging knowledge about the associations of these different morphotypes with different host niches and virulence potential, as well as the environmental cues and signalling pathways that are involved in the morphological transitions.
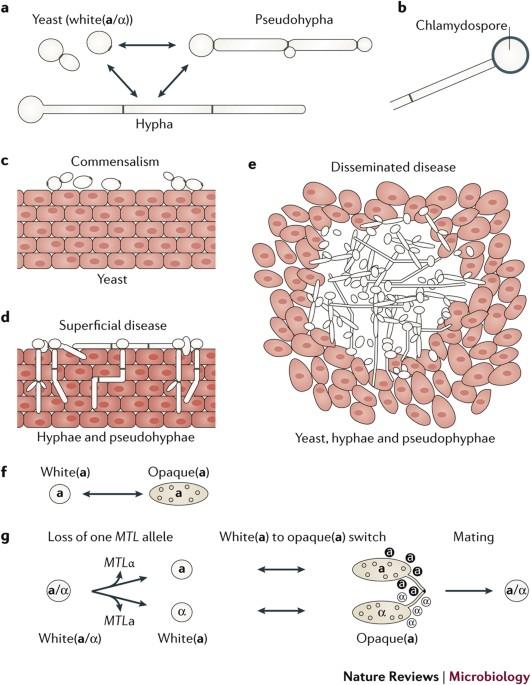
哺乳动物宿主体内的白色念珠菌细胞类型转换和功能可塑性
在这篇综述中,Noble 及其同事讨论了白色念珠菌经典细胞类型--酵母、菌丝、假菌丝和衣壳孢子--以及新发现的酵母样形态--包括灰色细胞和胃肠诱导转化(GUT)细胞类型--的特征,并重点介绍了它们与不同宿主生态位的关联以及致病性与共生性倾向方面的新知识。白色念珠菌是哺乳动物微生物组中无处不在的共生菌,也是人类最常见的真菌病原体。人们认为,白念珠菌在酵母和菌丝形态之间的细胞类型转变是在不同宿主组织中观察到的毒力差异的主要原因。然而,最近报道的新型酵母样细胞形态,包括不透明(a/α)、灰色和胃肠诱导转化(GUT)细胞类型,在体外和动物共生及疾病模型中表现出明显的差异。在本综述中,我们将探讨传统细胞类型(酵母、菌丝、假菌丝和衣壳孢子)以及新发现的酵母样形态的特征。我们将重点介绍这些不同形态与不同宿主生态位和毒力潜能之间的关系,以及形态转变所涉及的环境线索和信号通路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


