How protons pave the way to aggressive cancers
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
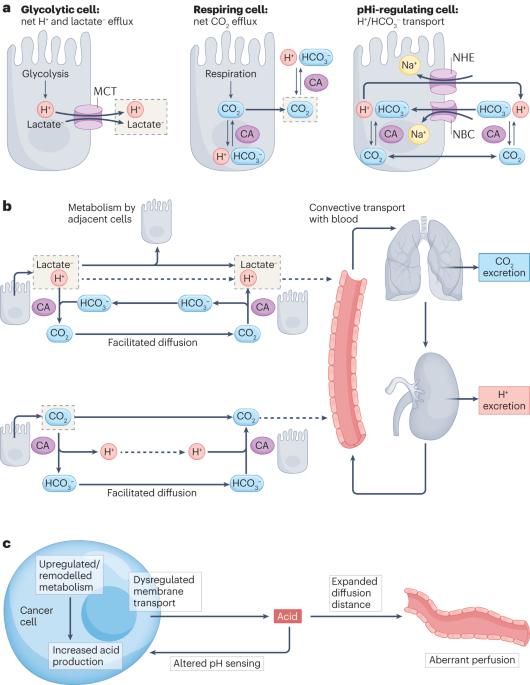
Cancers undergo sequential changes to proton (H+) concentration and sensing that are consequences of the disease and facilitate its further progression. The impact of protonation state on protein activity can arise from alterations to amino acids or their titration. Indeed, many cancer-initiating mutations influence pH balance, regulation or sensing in a manner that enables growth and invasion outside normal constraints as part of oncogenic transformation. These cancer-supporting effects become more prominent when tumours develop an acidic microenvironment owing to metabolic reprogramming and disordered perfusion. The ensuing intracellular and extracellular pH disturbances affect multiple aspects of tumour biology, ranging from proliferation to immune surveillance, and can even facilitate further mutagenesis. As a selection pressure, extracellular acidosis accelerates disease progression by favouring acid-resistant cancer cells, which are typically associated with aggressive phenotypes. Although acid–base disturbances in tumours often occur alongside hypoxia and lactate accumulation, there is now ample evidence for a distinct role of H+-operated responses in key events underpinning cancer. The breadth of these actions presents therapeutic opportunities to change the trajectory of disease. In this Review, Swietach and colleagues discuss how the pH balance is dysregulated in tumours and how alterations in intracellular and extracellular pH affect tumour biology to accelerate disease progression, providing a rationale for therapeutic targeting of acid–base disturbances in cancer.
质子如何为侵袭性癌症铺平道路。
癌症经历质子(H+)浓度和传感的连续变化,这是疾病的后果,并促进其进一步发展。质子化状态对蛋白质活性的影响可能来自氨基酸的改变或其滴定。事实上,许多引发癌症的突变以一种能够在正常限制之外生长和侵袭的方式影响pH平衡、调节或传感,作为致癌转化的一部分。当肿瘤由于代谢重编程和灌注紊乱而形成酸性微环境时,这些癌症支持作用变得更加突出。随之而来的细胞内和细胞外pH紊乱影响肿瘤生物学的多个方面,从增殖到免疫监测,甚至可以促进进一步的诱变。作为一种选择压力,细胞外酸中毒通过有利于耐酸癌症细胞而加速疾病进展,而耐酸细胞通常与侵袭性表型有关。尽管肿瘤中的酸碱紊乱通常与缺氧和乳酸积累同时发生,但现在有充分的证据表明,H+操作的反应在支撑癌症的关键事件中起着独特的作用。这些作用的广度提供了改变疾病发展轨迹的治疗机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


