A multi-targeted treatment approach to cancer cachexia: Auckland's Cancer Cachexia evaluating Resistance Training (ACCeRT) trial
Abstract
Background
Cancer cachexia is a condition often seen at diagnosis, throughout anti-cancer treatments and in end-stage non-small-cell lung cancer patients.
Methods and results
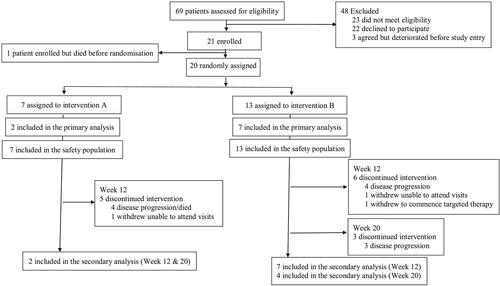
Participants with late-stage non-small-cell lung cancer and cachexia (defined as ≥5% weight loss within 12 months) were randomly assigned 1:2 to 2.09 g of eicosapentaenoic acid (EPA) and 300 mg of cyclo-oxygenase-2 inhibitor celecoxib orally once daily vs. same dosing of EPA, celecoxib, plus two sessions per week of progressive resistance training and 20 g of oral essential amino acids high in leucine in a split dose over 3 days, after each session. Primary endpoint was the acceptability of the earlier multi-targeted approach. Main secondary endpoints included change in body weight and fat-free mass, by bioelectric impedance analysis and total quadriceps muscle volume by magnetic resonance imaging over 20 weeks. Sixty-nine patients were screened resulting in 20 patients being enrolled. Acceptability scored high, with 4.5/5 (Arm A) and 5/5 (Arm B) for EPA and 5/5 for celecoxib within both arms and 4.8/5 for progressive resistance training sessions and 4.5/5 for essential amino acids within Arm B, all at Week 20. Results showed a net gain in bioelectric impedance analysis fat-free mass of +1.3 kg, n = 2 (Arm A), compared with +0.7 kg, n = 7 (Arm B) at Week 12, and —1.5 kg, n = 2 (Arm A), compared with —1.7 kg, n = 4 (Arm B) at Week 20. Trends in efficacy in terms of improvement and/or stability in cachexia markers were seen within magnetic resonance imaging muscle volume, albumin, and C-reactive protein levels within both arms. There were no exercise-related adverse events, with one possible related adverse event of asymptomatic atrial fibrillation in one participant within Arm A.
Conclusions
Non-small-cell lung cancer cachectic patients are willing to be enrolled onto a multi-targeted treatment regimen and may benefit from cachexia symptom management even during the late/refractory stage.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


