Improved spectral resolution of [13C,1H]-HSQC spectra of aromatic amino acid residues in proteins produced by cell-free synthesis from inexpensive 13C-labelled precursors
IF 1.9
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 1
Abstract
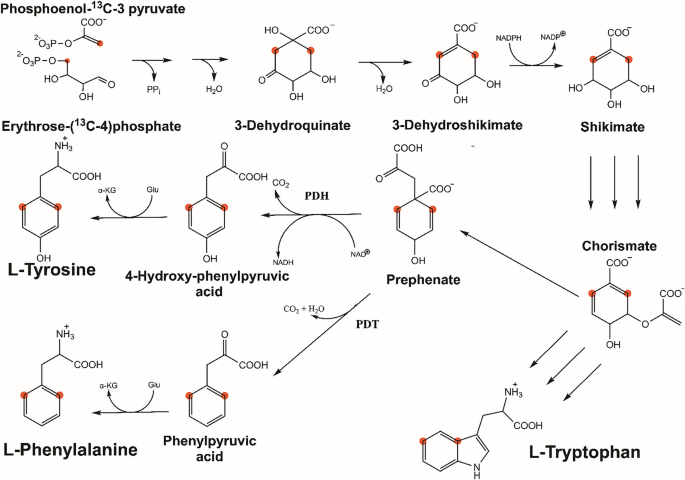
Cell-free protein synthesis using eCells allows production of amino acids from inexpensive 13C-labelled precursors. We show that the metabolic pathway converting pyruvate, glucose and erythrose into aromatic amino acids is maintained in eCells. Judicious choice of 13C-labelled starting material leads to proteins, where the sidechains of aromatic amino acids display [13C,1H]-HSQC cross-peaks free of one-bond 13C–13C couplings. Selective 13C-labelling of tyrosine and phenylalanine residues is achieved simply by using different compositions of the reaction buffers.
提高了用廉价的13C标记前体无细胞合成蛋白质中芳香氨基酸残基的[13C,1H]-HSQC光谱的光谱分辨率
使用eccells进行无细胞蛋白质合成,可以从廉价的13c标记前体中生产氨基酸。我们发现,在细胞中,丙酮酸、葡萄糖和红细胞转化为芳香氨基酸的代谢途径是维持的。明智地选择13C标记的起始材料可以生成蛋白质,其中芳香氨基酸侧链显示[13C,1H]-HSQC交叉峰,没有单键13C- 13C偶联。酪氨酸和苯丙氨酸残基的选择性13c标记可以通过使用不同的反应缓冲液来实现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Biomolecular NMR
生物-光谱学
CiteScore
6.00
自引率
3.70%
发文量
19
审稿时长
6-12 weeks
期刊介绍:
The Journal of Biomolecular NMR provides a forum for publishing research on technical developments and innovative applications of nuclear magnetic resonance spectroscopy for the study of structure and dynamic properties of biopolymers in solution, liquid crystals, solids and mixed environments, e.g., attached to membranes. This may include:
Three-dimensional structure determination of biological macromolecules (polypeptides/proteins, DNA, RNA, oligosaccharides) by NMR.
New NMR techniques for studies of biological macromolecules.
Novel approaches to computer-aided automated analysis of multidimensional NMR spectra.
Computational methods for the structural interpretation of NMR data, including structure refinement.
Comparisons of structures determined by NMR with those obtained by other methods, e.g. by diffraction techniques with protein single crystals.
New techniques of sample preparation for NMR experiments (biosynthetic and chemical methods for isotope labeling, preparation of nutrients for biosynthetic isotope labeling, etc.). An NMR characterization of the products must be included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


