Microwave-synthesized γ-WO3 nanorods exhibiting high current density and diffusion characteristics
Abstract
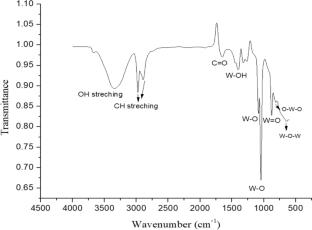
The synthesis of monoclinic (γ) tungsten oxide (WO3) nanorods via facile Microwave-assisted hydrothermal route is reported in the present work. The structural characterization of the as-synthesized material by using X-ray diffraction and Fourier-transform infrared spectroscopy confirms the formation of crystalline WO3 phase. The morphology and microstructural study along with elemental composition of the material as obtained by scanning electron microscopy and transmission electron microscopy, respectively, reveals the generation of one-dimensional WO3 nanorods. The nanorods show substantial absorbance in the ultraviolet (UV) region with the bandgap and refractive index of 2.7 eV and 2.48, respectively. Here, the low value of bandgap without adding any catalyst or co-catalyst is attributed to the microwave treatment. The electrochemical properties of WO3 are studied using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The nanorods show high current density at different potentials and diffusion-controlled behavior as exhibited by Warburg impedance. The anodic as well as cathodic peak current values are seen to be increased after the deposition of the thin film of FTO substrate indicating the better diffusion of ions in the electrolyte. The capacitive and diffusive contribution of the thin film is investigated at various scan rates using Dunn’s model which shows that the diffusive contribution of the thin film is 120 times more than the capacitive contribution confirming the diffusive behavior of the thin film. The exchange current density of the deposited film is calculated which is found to have higher value than that of bare FTO. I–V characteristics of WO3 are compared with that of bare FTO which reveals the smaller resistance offered by WO3 film. The equivalent circuit as obtained from Nyquist plot is used to estimate the resistance of electrolyte, film and charge transfer resistance along with the double-layer capacitance and Warburg impedance. Further, bode plot is analyzed to study the phase shift and thus the diffusive behavior of the material.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


