Three-dimensional printed Zn2SiO4/sodium alginate composite scaffold with multiple biological functions for tendon-to-bone repair
引用次数: 0
Abstract
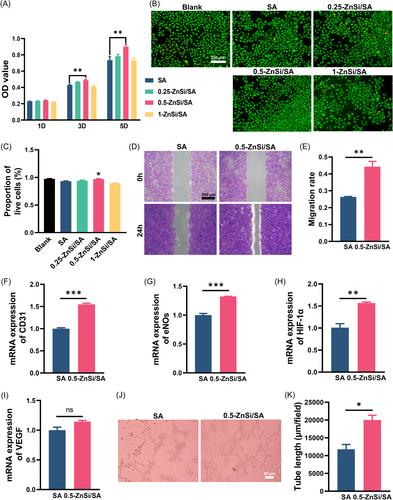
The intricate nature of the tendon–bone interface poses significant challenges for current surgical methods aimed at repairing tendon–bone interface injuries. Despite notable progress in surgical techniques, these methods continue to grapple with hurdles such as complications and suboptimal healing effects. In this study, we prepared a three‐dimensional‐printed composite scaffold by incorporating bioactive ceramic zinc silicate (Zn2SiO4) into sodium alginate (SA) hydrogel. The physicochemical properties and mechanical strength of the SA hydrogel scaffold composited with Zn2SiO4 (ZnSi/SA) were investigated in vitro. Impressively, the 0.5‐ZnSi/SA scaffold exhibited a sustained release of Zn and Si ions, while exhibiting mechanical properties compatible with tendon–bone interface repair. Moreover, cell viability, cell migration, and osteogenic differentiation assay results showed that 0.5‐ZnSi/SA scaffold facilitated the viability, mobility, and osteogenic differentiation of bone marrow stromal cells. In parallel, assessments of cell viability, cell migration, and tendon differentiation indicated that 0.5‐ZnSi/SA scaffold promoted the viability, migration, and tenogenic differentiation of tendon stem/progenitor cells. Moreover, cell viability, cell migration, and tube formation assay results demonstrated that 0.5‐ZnSi/SA scaffold enhanced the viability, migration rate, and angiogenic performance of human umbilical vein endothelial cells. Collectively, our findings suggest a promising therapeutic avenue employing ZnSi/SA scaffold for tendon–bone interface healing.
具有多种生物功能的Zn2SiO4/藻酸钠三维打印复合支架用于腱骨修复
腱骨界面的复杂性对目前旨在修复腱骨界面损伤的手术方法提出了重大挑战。尽管在外科技术方面取得了显著进展,但这些方法仍在努力克服并发症和次优愈合效果等障碍。在本研究中,我们将生物活性陶瓷硅酸锌(Zn2SiO4)掺入藻酸钠(SA)水凝胶中,制备了一种三维印刷复合支架。研究了Zn2SiO4(ZnSi/SA)复合SA水凝胶支架的物理化学性能和力学强度。令人印象深刻的是,0.5-ZnSi/SA支架表现出Zn和Si离子的持续释放,同时表现出与肌腱-骨界面修复兼容的机械性能。此外,细胞活力、细胞迁移和成骨分化测定结果表明,0.5-ZnSi/SA支架促进了骨髓基质细胞的活力、迁移和成骨性分化。同时,对细胞活力、细胞迁移和肌腱分化的评估表明,0.5-ZnSi/SA支架促进了肌腱干细胞/祖细胞的活力、迁移和腱分化。此外,细胞活力、细胞迁移和管形成测定结果表明,0.5-ZnSi/SA支架增强了人脐静脉内皮细胞的活力、迁移率和血管生成性能。总之,我们的研究结果表明,使用ZnSi/SA支架进行肌腱-骨界面愈合是一种很有前途的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


