Organophotoredox 1,6-Addition of 3,4-Dihydroquinoxalin-2-ones to para-Quinone Methides Using Visible Light
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An organophotoredox 1,6-radical addition of 3,4-dihidroquinoxalin-2-ones to para-quinone methides catalyzed by Fukuzumi’s photocatalyst is described under the irradiation of a HP Single LED (455 nm). The corresponding 1,1-diaryl compounds bearing a dihydroquinoxalin-2-one moiety (20 examples) are obtained with good to excellent yields under mild reaction conditions. Several experiments have been carried out in order to propose a reaction mechanism.
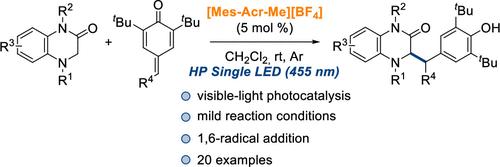
利用可见光将3,4-二氢喹啉-2-酮加成到对醌类化合物中
在HP单LED(455nm)的照射下,用Fukuzumi的光催化剂催化3,4-二氢喹啉-2-酮与对醌甲酰胺的有机光氧化还原1,6-自由基加成。在温和的反应条件下以良好至优异的产率获得相应的带有二氢喹喔啉-2-酮部分的1,1-二芳基化合物(20个实例)。为了提出反应机理,已经进行了几个实验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


