Electrochemical oxidation of sec-alcohols with MgBr2·6H2O
引用次数: 1
Abstract
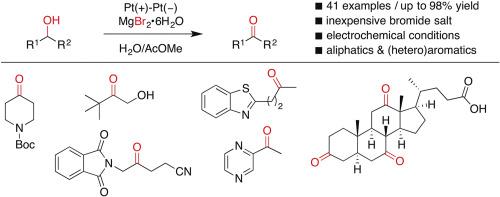
The electrochemical oxidation of sec-alcohols has been achieved using MgBr2·6H2O as an inexpensive active bromine source and electrolyte under constant current conditions. The reactions smoothly proceed in a simple undivided cell, and aliphatic/benzylic sec-alcohols bearing heteroaromatics as well as aryl and alkyl groups are successfully converted to the corresponding ketones in good to excellent yields. In addition, the present reaction conditions selectively transform a secondary hydroxy moiety over different classes of hydroxy groups.
二醇与MgBr2·6H2O的电化学氧化研究
用MgBr2·6H2O作为廉价的活性溴源和电解质,在恒流条件下实现了仲醇的电化学氧化。反应在一个简单的不可分割的单元中顺利进行,带有杂芳族以及芳基和烷基的脂族/苄基仲醇以良好至优异的产率成功转化为相应的酮。此外,本反应条件在不同类别的羟基上选择性地转化仲羟基部分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


