Synergistic combination of natural deep eutectic solvent and chemical vapor generation for trace determination of As, Cd, Hg and Pb in drug samples by inductively coupled plasma optical emission spectrometry
Abstract
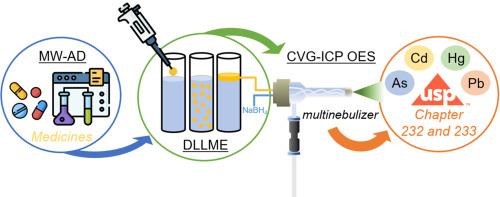
A new and environmentally friendly analytical method for simultaneous determination of As, Cd, Hg and Pb in drug samples by inductively coupled plasma optical emission spectrometry (ICP OES) has been developed. In order to increase the sensitivity of the analysis, a multinebulizer has been used for chemical vapor generation (CVG) after a dispersive liquid-liquid microextraction (DLLME) procedure using a natural deep eutectic solvent (NADES) as extractant solvent. The factors affecting analyte extraction and on-line chemical vapor generation have been optimized by multivariate analysis. Under optimized conditions, DLLME-CVG-ICP OES improved limit of quantitation (LOQ) values on average 50-fold higher compared with direct ICP OES analysis and afforded an increase of the sensitivity (i.e., enrichment factor) on average 25-times higher than those obtained with CVG-ICP OES analysis. According to the United States Pharmacopoeia (USP) Chapter 232, it means LOQ values are on average 4.3-times lower than their respective 0.3J values for the target-elements from class 1. Trueness has been evaluated by recovery experiments following USP recommendations for two oral drug samples in solid dosage form (i.e., commercial dosage tablets). In addition, the greenness of the developed method has been evaluated using the AGREEprep metrics, showing an excellent green character since it includes the miniaturization of the sample preparation procedure using a reduced volume (i.e., few microliters) of a non-hazardous extractant solvent for multielemental analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


