The enantioselective enhancing effect and mechanistic insights of chiral enhancers in transdermal drug delivery
Abstract
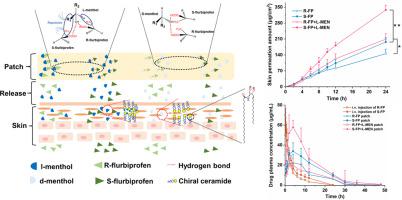
Overlook of chiral consideration in transdermal drug delivery increases administrated dose and risk of side effects, decreasing therapeutical effects. To improve the transdermal delivery efficiency of eutomer, this work focused on investigating the law and mechanism of enantioselective enhancing effects of chiral permeation enhancers on drug enantiomers. Chiral nonsteroidal anti-inflammatory drugs and terpene permeation enhancers were selected as model drug and enhancers. The results indicated that the L-isomer of permeation enhancers increased the skin absorption of S-enantiomer of drug and D-isomer improve the permeation of R-enantiomer, in which the enhancement effect (ER) of L-menthol on S-enantiomer (ER = 3.23) was higher than that on R-enantiomer (ER = 1.49). According to the pharmacokinetics results, L-menthol tended to enhance the permeation of S-enantiomer better than R-enantiomer (2.56 fold), and showed excellent in vitro/in vivo correlations. The mechanism study showed that L-isomer of permeation enhancers improved the permeation of S-enantiomer by increasing the retention, but the D-isomer by improving partition for better permeation. Enantioselective mechanism indicated that the weaker chiral H-bond interaction between drug-chiral enhancers was caused by the enantiomeric conformation. Additionally, stronger chiral enhancers-skin interaction between L-isomer and S-conformation of ceramide produced better enhancing effects. In conclusion, enantioselective interaction of chiral drug-chiral enhancers and chiral enhancers-chiral skin played a critical role in transdermal drug delivery, rational utilization of which contributed to improving the uptake of eutomer and inhibiting distomers to decrease a half of dose and side effects, increasing transdermal therapeutical efficiency.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


