Organic solvent-free synthesis of sulfonyl hydrazides in water
引用次数: 0
Abstract
An organic solvent-free synthesis of sulfonyl hydrazides has been achieved in water. The reactions of equimolar amount of sulfonyl chlorides and hydrazines afforded a series of substituted sulfonyl hydrazides at 60 °C for 1 h in water as a solvent in the presence of triethylamine when hydrazines are hydrochlorides. Moderate to good yields were observed for this transformation by the simple operation (46–93%). The reaction of amines, anilines, alcohol, and phenol have also been investigated in water.
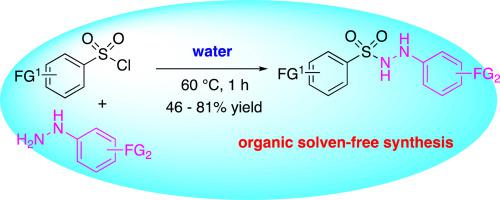
磺酰肼在水中的有机无溶剂合成
在水中实现了磺酰肼的无溶剂有机合成。等摩尔量的磺酰氯和肼的反应在60℃得到一系列取代的磺酰肼°C 1当肼是盐酸盐时,在三乙胺存在下在水中作为溶剂。通过简单操作观察到该转化的中等至良好产率(46–93%)。胺、苯胺、醇和苯酚在水中的反应也进行了研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


