Parafilm as an efficient transport matrix for corneal scrapings.
Q3 Medicine
引用次数: 0
Abstract
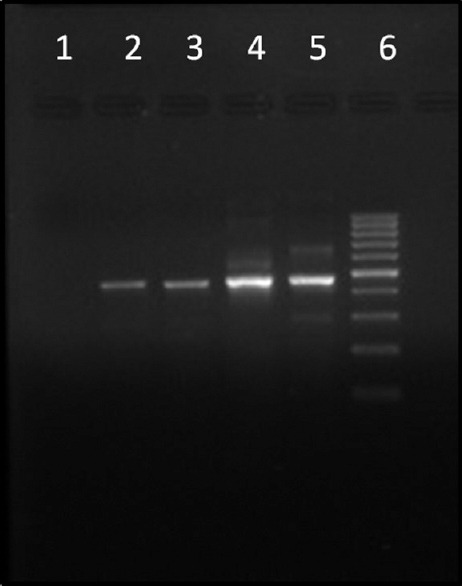
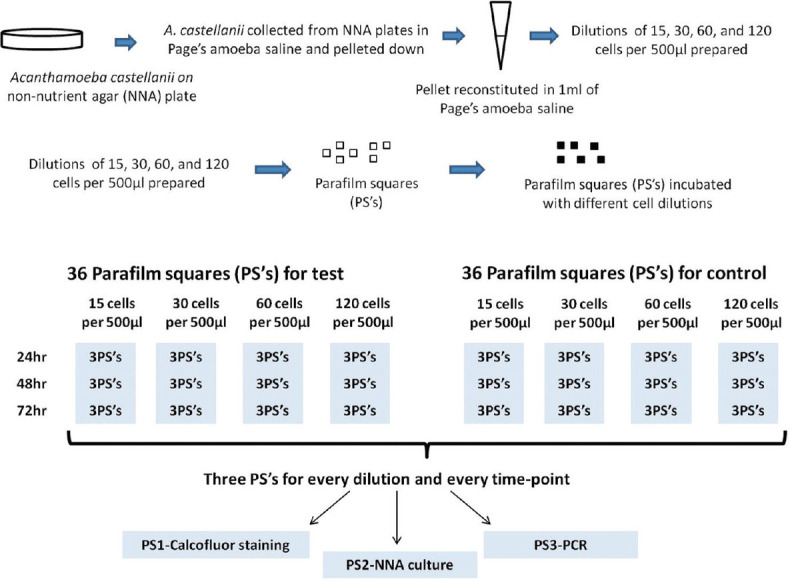
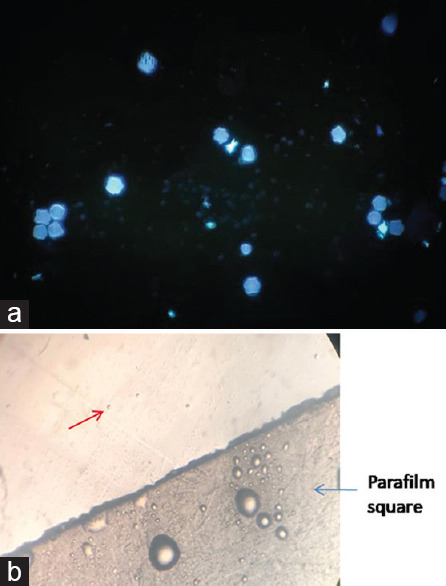
Introduction: Acanthamoeba spp. are free-living parasites increasingly implicated in causing Acanthamoeba keratitis (AK). AK is diagnosed by demonstration of parasites in corneal samples by direct microscopy, culture, and nucleic acid amplification. Most commonly, corneal scrapings are sent to the laboratory smeared between two glass slides. These scrapings are suitable for direct microscopy but less suitable for culture and polymerase chain reaction (PCR) which, in turn, are more sensitive for the diagnosis of AK. Aim: The aim of the study was to explore better alternatives for transporting corneal scrapings from the point-of-care eye center to the concerned laboratories. Materials and Methods: The study used small Parafilm (Bemis Company Inc., USA) squares (PSs) of 1 cm each prepared by cutting Parafilm using a surgical blade under sterile conditions. Each of the four different dilutions of Acanthamoeba suspension (15, 30, 60, and 120 cells) was used in this study. Each dilution was added onto the surface of 36 PSs and kept at room temperature for 24-h, 48-h, and 72-h incubation. The PSs for one particular time point and dilution were used for calcofluor white staining, its inoculation onto the surface of nonnutrient agar having a lawn of Escherichia coli, and Acanthamoeba-specific PCR amplification. In addition, two PSs inoculated with 30 cells and incubated for 24 h and 72 h were used for scanning electron microscopy (SEM). Results and Conclusion: All three diagnostic techniques, i.e. microscopy, culture, and PCR, detected the presence of Acanthamoeba at all the tested concentrations and time points. However, the growth pattern on culture changed directly in proportion to increased incubation periods and increased concentration of inoculum. In addition, the adherence of Acanthamoeba to the Parafilm was confirmed by SEM; these results suggest the use of these PSs as a suitable matrix for the transport of corneal scrapings.
Parafilm作为角膜刮伤的有效传输基质。
简介:棘阿米巴是一种自由生活的寄生虫,越来越多地与引起棘阿米巴角膜炎(AK)有关。AK是通过直接显微镜、培养和核酸扩增在角膜样本中显示寄生虫来诊断的。最常见的是,角膜刮片被送到实验室,涂抹在两张载玻片之间。这些刮片适用于直接显微镜检查,但不太适用于培养和聚合酶链式反应(PCR),后者反过来对AK的诊断更敏感。目的:本研究的目的是探索将角膜刮片从护理点眼科中心运送到相关实验室的更好替代方案。材料和方法:该研究使用1厘米的小Parafilm(Bemis Company股份有限公司,USA)正方形(PS),每个正方形通过在无菌条件下使用手术刀片切割Parafilm而制备。本研究中使用了四种不同稀释度的棘阿米巴悬浮液(15、30、60和120个细胞)。将每个稀释液添加到36个PS的表面上,并在室温下保持24小时、48小时和72小时孵育。使用一个特定时间点的PS和稀释液进行钙荧光白染色,将其接种到具有大肠杆菌草坪的非营养琼脂表面,并进行棘阿米巴特异性PCR扩增。此外,用30个细胞接种并孵育24小时和72小时的两个PS进行扫描电子显微镜(SEM)。结果和结论:显微镜、培养和PCR三种诊断技术在所有测试浓度和时间点都检测到棘阿米巴的存在。然而,培养物上的生长模式与培养时间的增加和接种物浓度的增加成正比。此外,扫描电镜证实了棘阿米巴对Parafilm的粘附性;这些结果表明使用这些PS作为运输角膜刮片的合适基质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tropical Parasitology
Medicine-Infectious Diseases
CiteScore
2.40
自引率
0.00%
发文量
12
期刊介绍:
Tropical Parasitology, a publication of Indian Academy of Tropical Parasitology, is a peer-reviewed online journal with Semiannual print on demand compilation of issues published. The journal’s full text is available online at www.tropicalparasitology.org. The journal allows free access (Open Access) to its contents and permits authors to self-archive final accepted version of the articles on any OAI-compliant institutional / subject-based repository. The journal will cover technical and clinical studies related to health, ethical and social issues in field of parasitology. Articles with clinical interest and implications will be given preference.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


