The efficacy and safety of beinaglutide alone or in combination with insulin glargine in Chinese patients with type 2 diabetes mellitus who are inadequately controlled with oral antihyperglycemic therapy: A multicenter, open-label, randomized trial
Abstract
Background
To compare glycemic control in Chinese patients with type 2 diabetes mellitus (T2DM) whose blood glucose levels were inadequately controlled with oral antidiabetic drugs after beinaglutide alone or combined with insulin glargine (IGlar).
Methods
In this 16-week multicenter, randomized clinical trial, 68 participants randomly received beinaglutide or IGlar for 8 weeks, then the two drugs in combination for 8 weeks. The primary outcomes were the proportion of individuals achieving their glycemic target and the change in glucose variability as measured with a continuous glucose monitoring system from baseline to 8 and 16 weeks.
Results
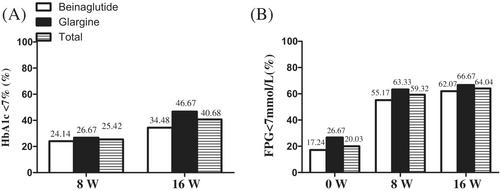
Both the beinaglutide and IGlar groups showed increased proportions achieving their glycemic target at 8 weeks, and the combination augmented the proportion reaching the glycated hemoglobin target from 25.42% at 8 weeks to 40.68% at 16 weeks. The beinaglutide group showed a significant reduction in body weight, body mass index, waist circumference, and systolic blood pressure. Beinaglutide elevated high-density lipoprotein cholesterol by 0.08 mmol/L (95% confidence interval [CI], 0.00–0.16), and diminished low-density lipoprotein cholesterol by 0.21 mmol/L (95% CI, 0.05–0.48), whereas IGlar showed no effect. Though IGlar was more efficient in lowering fasting plasma glucose than beinaglutide at comparable efficacies (to −1.57 mmol/L [95% CI, −2.60 to −0.54]), this difference was abolished in patients whose fasting C-peptide was ≥0.9 ng/mL.
Conclusion
Beinaglutide exhibited a favorable hypoglycemic effect on patients with T2DM, and in combination with IGlar, glucose level was further decreased. Low fasting C-peptide in patients may reduce the glycemic response to beinaglutide therapy. We recommend that C-peptide levels be evaluated when using or switching to the novel glucagon-like peptide-1 receptor agonists beinaglutide.
Trial Registration
ClinicalTrials.gov: NCT03829891.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


