Impact of chiglitazar on glycemic control in type 2 diabetic patients with metabolic syndrome and insulin resistance: A pooled data analysis from two phase III trials
Abstract
Background
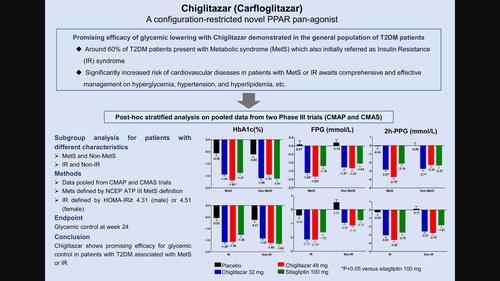
To evaluate the glycemic control effects of vhiglitazar (carfloglitazar), a novel peroxisome proliferator-activated receptor pan-agonist, in patients with type 2 diabetes mellitus (T2DM) with metabolic syndrome (MetS) or insulin resistance (IR) using pooled data analysis of two phase III clinical trials.
Methods
Data were collected from two randomized phase III clinical trials in China, comparing chiglitazar to placebo or sitagliptin in T2DM patients. The MetS was defined by the Adult Treatment Panel III MetS criteria, and IR was defined by homeostatic model assessment for insulin resistance (HOMA-IR) ≥4.31 (male) or 4.51 (female). The main end point of this analysis was glycemic control in the different arms within each subgroup.
Results
In the MetS subgroup, changes in glycated hemoglobin (HbA1c) from baseline at week 24 in the chiglitazar 32 mg, chiglitazar 48 mg, and sitagliptin 100 mg arms were −1.44%, −1.68%, and −1.37%, respectively; p < .05 was obtained when chiglitazar 48 mg was compared with sitagliptin. In the IR subgroup, the changes in HbA1c were −1.58%, −1.56%, and −1.26% in chiglitazar 32 mg, chiglitazar 48 mg, and sitagliptin 100 mg arms, respectively; p < .05 was obtained when chiglitazar 32 mg was compared with sitaligptin. The two doses of chiglitazar demonstrated a greater reduction in fasting plasma glucose and 2 h postprandial plasma glucose than sitagliptin in the pooled population and in the MetS and IR subgroups.
Conclusions
Chiglitazar shows promising efficacy for glycemic control in patients with T2DM associated with MetS or IR. Further prospective trials are required to validate these findings.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


