H2-reduced phosphomolybdate promotes room-temperature aerobic oxidation of methane to methanol
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The selective partial oxidation of methane to methanol using molecular oxygen (O2) represents a long-standing challenge, inspiring extensive study for many decades. However, considerable challenges still prevent low-temperature methane activation via the aerobic route. Here we report a precipitated Pd-containing phosphomolybdate, which, after activation by molecular hydrogen (H2), converts methane and O2 almost exclusively to methanol at room temperature. The highest activity reaches 67.4 μmol gcat−1 h−1. Pd enables rapid H2 activation and H spillover to phosphomolybdate for Mo reduction, while facile O2 activation and subsequent methane activation occur on the reduced phosphomolybdate sites. Continuous production of methanol from methane was also achieved by concurrently introducing H2, O2 and methane into the system, where H2 assists in maintaining a moderately reduced state of phosphomolybdate. This work reveals the underexplored potential of such a Mo-based catalyst for aerobic methane oxidation and highlights the importance of regulating the chemical valence state to construct methane active sites. The partial oxidation of methane to methanol is a very attractive yet challenging process. Now, a H2-reduced Pd-containing phosphomolybdate catalyst is reported to convert methane and O2 to methanol with nearly 100% selectivity at room temperature.
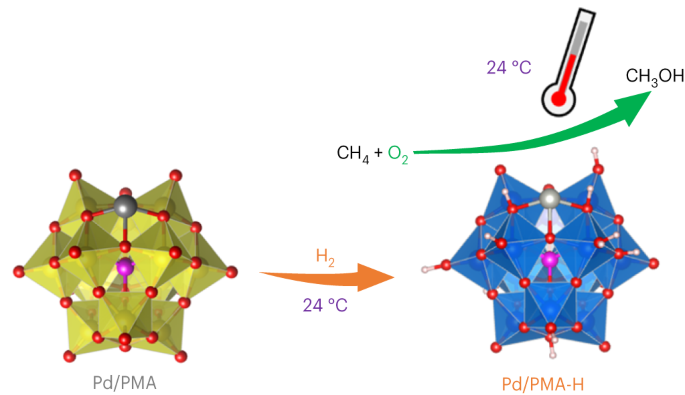
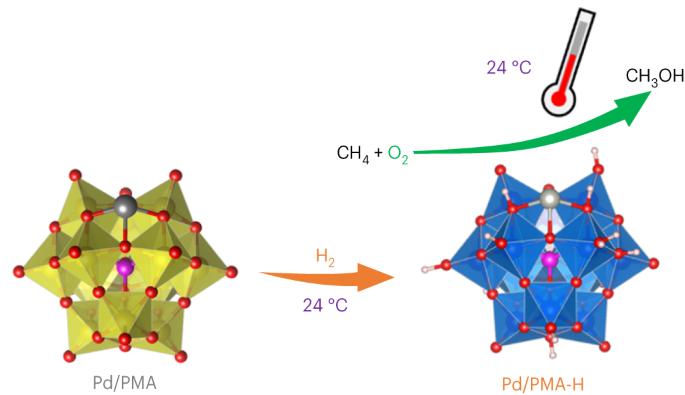
H2还原磷钼酸盐促进甲烷室温好氧氧化制甲醇
利用分子氧(O2)将甲烷选择性部分氧化为甲醇是一项长期存在的挑战,几十年来一直激励着人们进行广泛的研究。然而,通过有氧途径进行低温甲烷活化仍面临相当大的挑战。在此,我们报告了一种沉淀的含钯磷钼酸盐,它在被分子氢(H2)活化后,可在室温下将甲烷和 O2 几乎全部转化为甲醇。最高活性达到 67.4 μmol gcat-1 h-1。钯能使 H2 快速活化,并使 H 溢出到磷钼酸盐上,从而使 Mo 还原,而 O2 的活化和随后的甲烷活化则发生在磷钼酸盐的还原位点上。通过同时向系统中引入 H2、O2 和甲烷,还实现了从甲烷中连续生产甲醇,其中 H2 有助于维持磷钼酸盐的适度还原状态。这项研究揭示了这种钼基催化剂在有氧甲烷氧化方面尚未被充分开发的潜力,并强调了调节化合价态以构建甲烷活性位点的重要性。将甲烷部分氧化为甲醇是一个极具吸引力但又极具挑战性的过程。据报道,一种含 H2- 还原钯的磷钼酸盐催化剂可在室温下将甲烷和 O2 转化为甲醇,选择性接近 100%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


