Kinetics of Low-Temperature Conversion of Carbon Monoxide with Water Vapor on Cu-Zn-Al Oxide Catalysts
IF 0.7
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
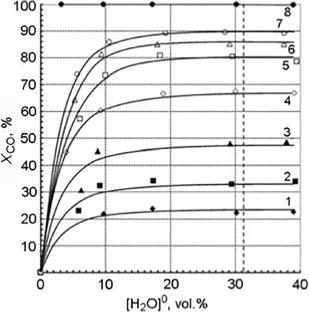
According to the analysis of literature data and our experimental results, a kinetic equation for the low-temperature conversion of carbon monoxide with water vapor over a highly active copper–zinc–aluminum oxide catalyst is proposed. It is shown that the obtained kinetic parameters can be used to calculate the reaction rate and optimize the process.
Cu-Zn-Al氧化物催化剂上一氧化碳与水蒸气低温转化动力学
根据文献资料和实验结果的分析,提出了高活性铜锌铝氧化物催化剂上一氧化碳与水蒸气低温转化的动力学方程。结果表明,所得动力学参数可用于计算反应速率和优化工艺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


