Polaron States of 3,4-Ethylenedioxythiophene Oligomers
IF 0.7
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electronic and spatial structure of 3,4-ethylenedioxythiophene oligomer containing 12 units (E12) in 0, +1, +2, +3, and +4 charge states has been calculated by the method of density functional theory (B3LYP 6-31 G**). It is shown that electron conductivity in the oxidized oligomer E124+ is provided by two bipolarons at the ends of the chain.
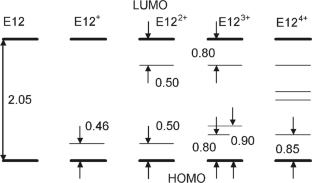
3,4-乙烯二氧噻吩低聚物的极化子态
采用密度泛函理论(B3LYP 6-31 G**)计算了含12个单元的3,4-乙烯二氧噻吩低聚物(E12)在0、+1、+2、+3和+4电荷态的电子结构和空间结构。结果表明,氧化低聚物E124+的电子导电性是由链两端的两个双极化子提供的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


