Iodobenzene-catalyzed photochemical heteroarylation of alcohols by rupture of inert C–H and C–C bonds
引用次数: 0
Abstract
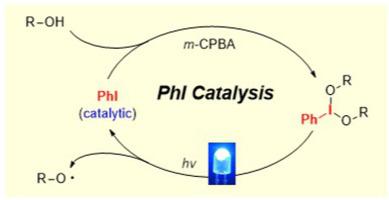
A Minisci-type reaction catalyzed by iodobenzene is disclosed here for the first time. The heteroarylation of unprotected aliphatic alcohols proceeds via alkoxy radical-induced homolytic cleavage of C–H and C–C bonds under photochemical conditions. The use of m-CPBA as the oxidant allows the oxidation of iodobenzene to a hypervalent iodine species, driving the catalytic cycle. The method features mild reaction conditions, broad scope of heteroarenes and alcohols, and scaled up preparations. This approach provides a notable supplement to iodobenzene-catalyzed ionic reactions, and opens up a new avenue for its application in radical chemistry.
通过破坏惰性碳氢键和碳碳键,碘苯催化醇的光化学异芳化反应
本文首次公开了一种由碘苯催化的微型反应。在光化学条件下,无保护的脂肪醇的异芳基化是通过烷氧自由基诱导的C-H和C-C键的均裂裂解进行的。使用m-CPBA作为氧化剂,允许将碘苯氧化为高价碘,驱动催化循环。该方法反应条件温和,杂环芳烃和醇类反应范围广,制备规模大。该方法为碘苯催化离子反应提供了有益的补充,为其在自由基化学中的应用开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


