Contemporary approaches to site-selective protein modification
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 218
Abstract
Proteins constitute the majority of nature’s worker biomolecules. Designed for specific functions, complex tertiary structures make proteins ideal candidates for analysing natural systems and creating novel biological tools. Owing to both their large size and the need for proper folding, de novo synthesis of proteins has been quite a challenge, leading scientists to focus on modifying protein templates already provided by nature. Recently developed methods for protein modification fall into two broad categories: those that can modify the natural protein template directly and those that require genetic manipulation of the amino acid sequence before modification. The goal of this Review is not only to provide a window through which to view the many opportunities created by novel protein modification techniques‚ but also to act as an initial guide to help scientists find direction and form ideas in an ever-growing field. In addition to highlighting methods reported in the past 5 years, we aim to provide a broader sense of the goals and outcomes of protein modification and bioconjugation in general. While the main body of this paper comprises reactions involving the direct modification of expressed proteins, some further functionalization strategies as well as biological applications are also acknowledged. The discussion concludes by speculating which trends and discoveries will most likely come next in the field. Over the past 5 years, many novel site-selective protein modification techniques have been reported. Key features of these various strategies as well as prominent examples are discussed in this Review.
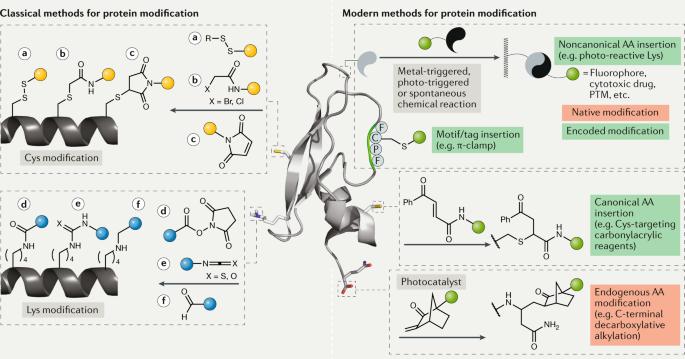
位点选择性蛋白质修饰的现代方法
蛋白质是大自然中的大多数生物大分子。蛋白质专为特定功能而设计,其复杂的三级结构使其成为分析自然系统和创造新型生物工具的理想候选物。由于蛋白质体积庞大且需要适当折叠,从头合成蛋白质一直是一个相当大的挑战,因此科学家们把重点放在了改造自然界已经提供的蛋白质模板上。最近开发的蛋白质修饰方法可分为两大类:一类是可直接修饰天然蛋白质模板的方法,另一类是在修饰前需要对氨基酸序列进行基因操作的方法。本综述的目的不仅在于提供一扇窗口,让人们了解新型蛋白质修饰技术所创造的众多机会,还在于提供一个初步指南,帮助科学家在这个不断发展的领域中找到方向、形成想法。除了重点介绍过去 5 年中报道的方法外,我们还旨在从更广泛的角度介绍蛋白质修饰和生物共轭的总体目标和成果。本文的主要内容包括对表达蛋白进行直接修饰的反应,同时也介绍了一些进一步的功能化策略和生物应用。讨论的最后还推测了该领域下一步最有可能出现的趋势和发现。过去 5 年中,许多新颖的位点选择性蛋白质修饰技术被报道出来。本综述讨论了这些不同策略的主要特点和突出实例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


