Chemoselective borylation of bromoiodoarene in continuous flow: synthesis of bromoarylboronic acids
IF 2
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 1
Abstract
The chemoselective borylation of bromoiodoarenes under continuous flow conditions is described. Bromoarylboronic acids were synthesized in high yields using iPrMgCl•LiCl as the halogen-metal exchange reagent, with 5.7–86 s residence time at room temperature. The process is compatible with a variety of functional groups, including carbanion-reactive moieties, enabling post-product functionalization. A gram-scale reaction, including a continuous-flow work-up, afforded the desired product in a highly efficient manner. Successful telescoping with a Suzuki coupling has also been performed, converting 1-bromo-3-iodobenzene to 3-bromo-1,1′-biphenyl on a gram-scale in a good yield.
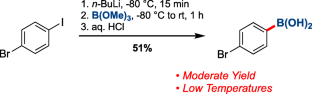
连续流动中溴碘芳烃的化学选择性硼化反应:溴芳基硼酸的合成
介绍了连续流动条件下溴碘芳烃的化学选择性硼化反应。以iPrMgCl•LiCl为卤素-金属交换试剂,高产率合成了溴基硼酸,室温停留时间为5.7 ~ 86 s。该工艺与多种官能团兼容,包括碳反应性基团,使产品后功能化。一个克级的反应,包括连续流动的处理,以高效率的方式提供所需的产品。通过铃木偶联,成功地将1-溴-3-碘苯以克为单位转化为3-溴-1,1 ' -联苯,收率很高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Flow Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
6.40
自引率
3.70%
发文量
29
审稿时长
>12 weeks
期刊介绍:
The main focus of the journal is flow chemistry in inorganic, organic, analytical and process chemistry in the academic research as well as in applied research and development in the pharmaceutical, agrochemical, fine-chemical, petro- chemical, fragrance industry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


