Stability Study and Simultaneous determination of Norepinephrine, Moxifloxacin, and Piperacillin + Tazobactam Mixtures applied in Intensive Care Medicine
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In intensive care units intravenous medicine may be used in simultaneous infusion in the same intravenous site. Sometimes, the physical compatibility and stability of the combined solutions are unknown. The objective was to develop, optimize and validate a simple, fast and sensitive stability-indicating high-performance liquid chromatography (HPLC) for simultaneous quantification of binary mixtures of norepinephrine, piperacillin + tazobactam, moxifloxacin for intravenous (IV) administration in different diluents and physical compatibility with mannitol. The HPLC method was performed on a C18LUNA (4.6x250 mm 5-Micron) column, using acetonitrile: methanol: phosphate buffer pH 3.0 (20:30:50) as eluent and validated according to ICH guidelines and applied to mixtures of norepinephrine, moxifloxacin, piperacillin, tazobactam and mannitol at 0, 2, 6, 9 and 24 h. The substances and their mixtures were also evaluated by visual inspection and pH over time. The analytical method developed was specific, linear, precise, accurate and robust. No visual changes were observed in the mixtures over time, maintaining the pH values (except for piperacillin + tazobactam which changed 0.5 in 24 h) and losses of less than 10% of content over the 24 h under analyzed conditions. The proposed method is suitable for simultaneous analysis of norepinephrine, moxifloxacin, piperacillin and tazobactam. All tested mixtures were compatible and stable for up to 24 h, which is an important result for increasing patient safety in clinical practice since it has not been reported in the literature yet. The method can be further investigated and used for different concentration and diluent combinations. Conclusion: The proposed method is suitable for simultaneous analysis of norepinephrine, moxifloxacin, piperacillin and tazobactam. All tested mixtures were compatible and stable for up to 24 h, which is an important result for increase patient safety in clinical practice, since it has not been reported in literature yet. The method can be further investigated and used for different concentration and diluents combinations. HPLC
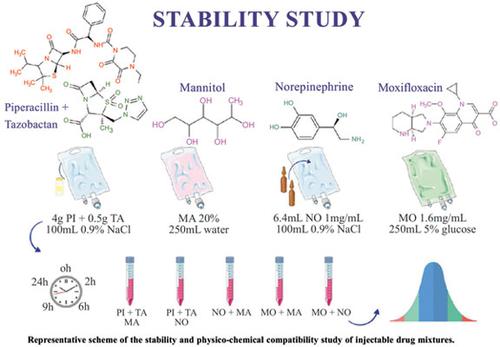
重症医学中去甲肾上腺素、莫西沙星、哌拉西林+他唑巴坦合剂的稳定性研究及同时测定
在重症监护病房,静脉药物可以在同一静脉部位同时输注。有时,组合溶液的物理相容性和稳定性是未知的。目的:建立、优化并验证一种简便、快速、灵敏且具有稳定性指示的高效液相色谱法(HPLC),用于同时定量不同稀释度的去甲肾上腺素、哌拉西林+他唑巴坦、莫西沙星静脉注射用二元混合物及其与甘露醇的物理相容性。HPLC法采用C18LUNA (4.6x250 mm 5微米)色谱柱,乙腈:甲醇:磷酸盐缓冲液pH 3.0(20:30:50)为洗脱液,根据ICH指南进行验证,并适用于去甲肾上腺素、莫西沙星、哌拉西林、他唑巴坦和甘露醇的混合物,分别在0、2、6、9和24 h。通过目测和pH随时间的变化对物质及其混合物进行评价。所建立的分析方法具有专属性、线性、精密度、准确度和鲁棒性。随着时间的推移,没有观察到混合物的视觉变化,保持pH值(除了哌拉西林+他唑巴坦在24小时内变化0.5),在分析条件下,24小时内含量损失小于10%。该方法适用于去甲肾上腺素、莫西沙星、哌拉西林和他唑巴坦的同时分析。所有测试的混合物在长达24小时内均具有相容性和稳定性,这是提高临床实践中患者安全性的重要结果,因为尚未有文献报道。该方法可以进一步研究,并用于不同的浓度和稀释剂组合。结论:该方法适用于去甲肾上腺素、莫西沙星、哌拉西林和他唑巴坦的同时分析。所有测试的混合物在长达24小时内都是相容和稳定的,这是在临床实践中提高患者安全性的重要结果,因为尚未有文献报道。该方法可以进一步研究,并用于不同的浓度和稀释剂组合。高效液相色谱法
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


