Adsorption of arsenic in aqueous solution onto iron impregnated bagasse fly ash
Abstract
Abstract
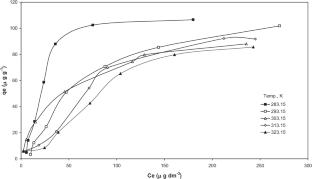
The present study examined the adsorption of As(III) and As(V) (arsenics) from aqueous solutions using FeCl3 impregnated bagasse fly ash (BFA-Fe). Batch adsorption studies were carried out to evaluate the effect of various parameters like initial pH (pH0), adsorbent dose (m), contact time (t), initial concentration (C0) and temperature (T) on the removal of As(III) and As(V) from aqueous solutions. The maximum removal of As(III) and As(V) was found ~ 95% and ~ 97% at lower concentration (< 20 μg/dm3) and ~ 86% and ~ 87% at higher concentration (500 μg/dm3), respectively, using 3 g/dm3 of BFA dosage at 303 K. The adsorption of arsenics on BFA-Fe was very rapid. Pseudo-second-order kinetic model well represented the adsorption kinetics of both As(III) and As(V). Error analyses functions for adsorption of As(III) and As(V) onto BFA-Fe. Based on these error analyses, R-P isotherm was found to be fitted. Thermodynamic parameters, i.e., ΔG°, ΔH°, and ΔS°, were also calculated. At 25.0 to 45.0 °C, the values of ΔG° lie in the range of -43.85, -45.34, -48.82, -51.31, -53.8, and -44.75, -48.3, -51.84, -55.39, -58.93, -55.57 for As (III), and As (V) respectively, indicating that adsorption is spontaneous and exothermic in nature. Regeneration study was carried out by different solvent and thermal methods. Our results revealed that BFA-Fe can be reused directly for making fire-briquettes to explore its energy value. From this study, As containment is most effective removal from aqueous solution and mimic to any contaminated water resources.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


