Accurate determination of the kinetics of toluene nitration in a liquid–liquid microflow system
Abstract
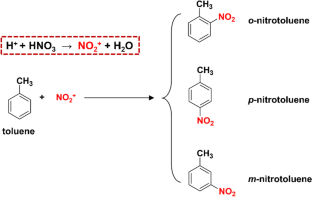
The nitration of toluene with mixed acid is one of the most representative nitration reactions. Accurate kinetic study is essential for controlling the reaction and designing reactors. Due to the characteristics of fast rate, high exothermicity and heterogeneity in toluene nitration, the effects of mass and heat transfer may result in inaccurate determination of kinetics. In this work, the adiabatic temperature rises of the reaction system were studied to provide precise ranges of experimental conditions for accurately controlling the reaction rate and heat release rate in a liquid–liquid microflow system. The adiabatic temperature rise was successfully controlled to below 0.3 °C. The effects of mass and heat transfer on the reaction rate were completely eliminated, so that the kinetic study was carried out under the control of intrinsic kinetics only. The activation energy for toluene nitration was determined to be 28.00 kJ/mol. The activation energies for the formation of o-nitrotoluene and p-nitrotoluene were obtained for the first time, which were 25.71 and 31.91 kJ/mol, respectively. The obtained kinetic models can predict the reaction performance of toluene nitration very well.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


