A Chitosan Hydrochloride Mediated, Simple and Efficient Approach for the Synthesis of Hydrazones, their in vitro Antimycobacterial Evaluations, and Molecular Modeling Studies (Part III)
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
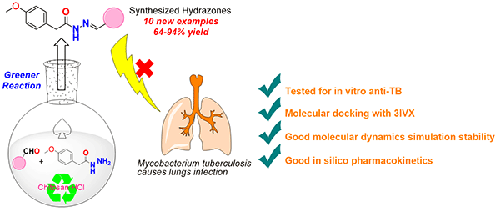
A simple, eco-friendly and straightforward synthesis of hydrazones have been devised in the presence of Chitosan Hydrochloride as catalyst in aqueous-ethanol medium at room temperature. The current protocol offers metal-free synthesis, adaptability to large-scaleup, good yields, and quicker reaction time. All synthesized hydrazones (3a-3j) were also depicted good antimycobacterial activity with MICs (minimum inhibitory concentrations) ranging from 3.12-6.25 µg/mL. Compound 3b represented strong binding affinity against M. tuberculosis pantothenate synthetase (pdb id: 3IVX) with a Glide docking score of -8.803 kcal/mol. Molecular dynamics simulation analysis of the complex 3b:3IVX retained good stability over the simulation period of 20 ns.
壳聚糖盐酸盐介导的简单有效的腙合成方法、体外抗菌评价和分子模型研究(第三部分)
在室温下,以壳聚糖盐酸盐为催化剂,在乙醇水介质中合成了一种简单、环保、直接的腙。目前的方案提供了无金属合成、大规模放大的适应性、良好的产率和更快的反应时间。所有合成的腙(3a-3j)也显示出良好的抗分枝杆菌活性,MIC(最小抑制浓度)范围为3.12-6.25µg/mL。化合物3b表现出对结核分枝杆菌泛酸合成酶(pdb-id:3IVX)的强结合亲和力,Glide对接得分为-8.803 kcal/mol。配合物3b:3IVX的分子动力学模拟分析在20ns的模拟周期内保持了良好的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


