Can an Amine Be a Weaker and a Stronger Base at the Same Time? Curious Cases of Chameleonic Ionization
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
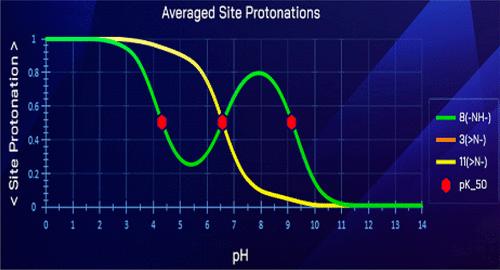
We discovered an anomalous basic dissociation in certain multiprotic compounds. An amine group placed in the middle of a given compound is predicted to behave unusually─at certain pH ranges, its averaged degree of protonation actually increases with pH (!) resulting from interactions with other ionizable groups. This chameleonic behavior results in two pK50 values: one corresponding to a weaker base and the other to a stronger base for the same group.
胺能同时成为弱碱和强碱吗?变色龙电离的奇怪案例
我们在某些多质子化合物中发现了反常的碱性解离。在特定的pH值范围内,由于与其他可电离基团的相互作用,它的平均质子化程度实际上随着pH值的增加而增加。这种变色龙行为导致两个pK50值:一个对应于较弱的碱基,另一个对应于同一群体的较强的碱基。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


