Fast anisotropic Mg and H diffusion in wet forsterite
Abstract
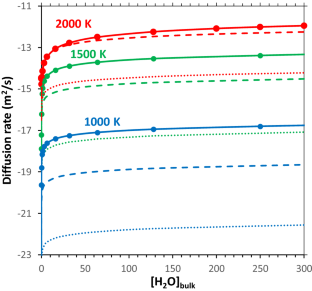
Adding hydrogen to forsterite strongly increases the diffusion rate of Mg, but the reason for this is unclear. As Mg diffusion in forsterite can influence its electrical conductivity, understanding this process is important. In this study we use density functional theory to predict the diffusivity of H-bearing Mg vacancies and we find that they are around 1000 times slower than H-free Mg vacancies. H-bearing Mg vacancies are many orders of magnitude more concentrated than H-free Mg vacancies, however, and diffusion is a combination of diffusivity and defect concentration. Overall, the presence of hydrated Mg vacancies is predicted to cause a large (multiple orders of magnitude) increase in both diffusion rate and diffusional anisotropy with a strong preference for diffusion in the [001] direction predicted. In models of experimental data, the effect of water concentration on diffusion is often described by a constant best-fitting exponent. Our results suggest that this exponent will vary between 0.5 and 1.6 across common experimental conditions with pressure decreasing and temperature increasing this exponent. These results suggest that Mg diffusion in forsterite could vary considerably throughout upper mantle conditions in ways that cannot be captured with a simple single-exponent model. Comparisons to measures of hydrogen diffusivity suggest that the diffusion of hydrated Mg vacancies also controls the diffusion of hydrogen in (iron-free) forsterite and that our conclusions above also apply to hydrogen diffusion rates and anisotropy. We also find that cation diffusivity likely cannot explain experimental measurements of the effect of water on electrical conductivity in olivine.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


