Unravelling the role of boron dopant in borocarbonitirde catalytic dehydrogenation reaction
Abstract
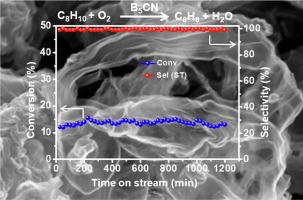
Borocarbonitride (BCN) materials are newly developed metal-free catalytic materials exhibiting high selectivity in oxidative dehydrogenation (ODH) of alkanes. However, the in-depth understandings on the role of boron (B) dopants and the intrinsic activities of –C=O and –B–OH still remain unknown. Herein, we report a series of BCN materials with regulable B content and surface oxygen functional groups via self-assembly and pyrolysis of guanine and boric acid. We found that the B/C ratio is the key parameter to determine the activity of ODH and product distribution. Among them, the high ethylbenzene conversion (∼57%) and styrene selectivity (∼83%) are achieved in ODH for B1CN. The styrene selectivity can be improved by increasing of B/C ratio and this value reaches near 100% for B5CN. Structural characterizations and kinetic measurements indicate that –C=O and –B–OH dual sites on BCN are real active sites of ODH reaction. The intrinsic activity of –C=O (5.556 × 10−4 s−1) is found to be 23.7 times higher than –B–OH (0.234 × 10−4 s−1) site. More importantly, we reveal that the deep oxidation to undesirable CO2 occurs on –C=O rather than –B–OH site, and B dopant in BCN materials can reduce the nucleophilicity of –C=O site to eliminate the CO2 emission. Overall, the present work provides a new insight on the structure–function relationship of the BCN catalytic systems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


