Alkali ions secure hydrides for catalytic hydrogenation
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 95
Abstract
Catalytic hydrogenation is one of the backbones of the chemical industry. Controlling the reaction behaviour of the activated hydrogen species over oxide-supported metal catalysts is essential. Aside from the expected addition to substrates, the activated hydrogen species would also destroy the active structures. Here we show that, with the assistance of alkali cations, the atomically dispersed Ru(iii) on Al2O3 exhibits enhanced performance in the hydrogenation of a broad range of substrates. The alkali cations facilitate the hydrogenation mediated by heterolytic hydrogen species, which not only restrain the hydride species from migrating to interfacial oxygen, thus suppressing the reduction and aggregation of ruthenium, but also stabilize the negatively charged transition states and intermediates through enhanced Columbic attraction. Distinctively, an inverse H/D isotope effect related to H2 splitting as the rate-determining step over the atomically dispersed ruthenium-catalysed hydrogenation is predicted and confirmed. Alkali metals have been traditionally used to promote heterogeneous catalysts, albeit their mode of action remains controversial. Now, the authors demonstrate the multifaceted role of sodium ions in promoting atomically dispersed Ru(iii) on Al2O3, resulting in a superior hydrogenation catalyst.
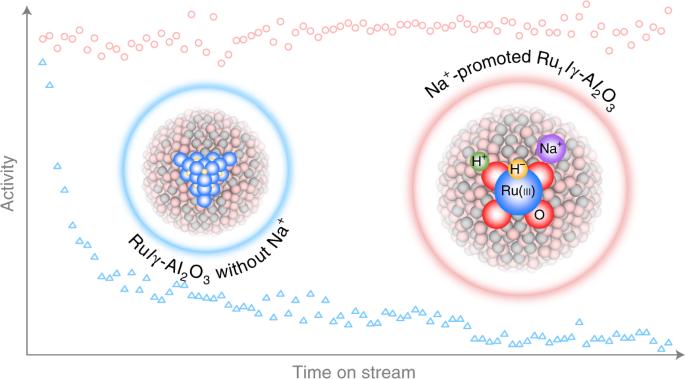
用于催化加氢的碱离子安全氢化物
催化氢化是化学工业的支柱之一。控制活性氢在氧化物支撑金属催化剂上的反应行为至关重要。除了对底物的预期加成之外,活化氢物种还会破坏活性结构。在这里,我们展示了在碱阳离子的帮助下,Al2O3 上原子分散的 Ru(iii) 在多种底物的氢化过程中表现出更高的性能。碱阳离子促进了异溶解氢物种介导的氢化反应,不仅抑制了氢化物物种向界面氧迁移,从而抑制了钌的还原和聚集,还通过增强的哥伦布吸引力稳定了带负电荷的过渡态和中间产物。与众不同的是,在原子分散的钌催化加氢过程中,H/D 同位素反向效应与作为速率决定步骤的 H2 分裂有关,这种效应得到了预测和证实。碱金属历来被用于促进异相催化剂,尽管其作用模式仍存在争议。现在,作者证明了钠离子在促进原子分散的 Ru(iii) 在 Al2O3 上的氢化过程中的多方面作用,从而产生了一种优异的氢化催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


