NHC-catalyzed regiodivergent transformations of Ynones with trifluoromethyl ketones: Aldol reaction or [3+2] annulation
引用次数: 0
Abstract
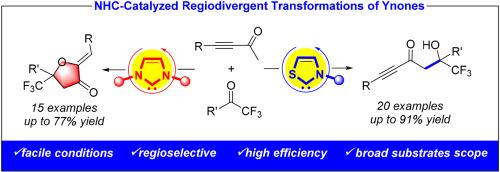
A novel NHC-catalyzed regiodivergent reaction of ynones with trifluoromethyl ketones consisting of Aldol reactions and intermolecular [3 + 2] annulations has been developed. Employing two different NHC catalysts, various tertiary alcohols and dihyrofuranones containing trifluoromethyl can be obtained in moderate to good yields (35 examples, up to 91% yield). This protocol features mild reaction conditions, high efficiency and broad substrates scope, promoting the development of NHC catalysis and transformations of ynones.
NHC催化Ynones与三氟甲基酮的区域收敛转化:Aldol反应或[3+2]环化
提出了一种新的nhc催化的壬酮与三氟甲基酮的区域发散反应,该反应由Aldol反应和分子间[3 + 2]环组成。采用两种不同的NHC催化剂,可获得各种叔醇和含三氟甲基的二氢呋喃酮,产率中等至较高(35例,产率高达91%)。该方案具有反应条件温和、效率高、底物范围广等特点,促进了NHC催化和炔酮转化的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


