Photoredox-Catalyzed Cascade of o-Hydroxyarylenaminones to Access 3-Aminated Chromones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 12
Abstract
Reported herein is a photoredox-catalyzed amination of o-hydroxyarylenaminones with tert-butyl ((perfluoropyridin-4-yl)oxy)carbamate, a versatile amidyl-radical precursor developed in our laboratory. This work establishes a new cascade pathway for the assembly of a range of 3-aminochromones under mild conditions. Downstream transformations of the obtained 3-aminochromones to construct diverse amino pyrimidines greatly broaden the applications of this photocatalyzed protocol.
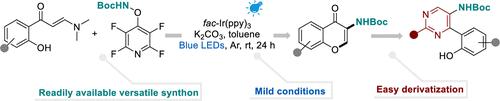
光氧化还原催化的邻羟基亚胺级联获得3-胺化色素
本文报道了一种光氧化催化的邻羟基亚胺与叔丁基((全氟吡啶-4-基)氧氨基甲酸酯的胺化反应,叔丁基(全氟吡啶-4-基)氨基甲酸酯是我们实验室开发的一种多功能的酰胺基前体。这项工作为一系列3-氨基色素在温和条件下的组装建立了新的级联途径。将得到的3-氨基色素下游转化为多种氨基嘧啶,大大拓宽了这种光催化方案的应用范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


