Global targeting of functional tyrosines using sulfur-triazole exchange chemistry
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 8
Abstract
Covalent probes serve as valuable tools for global investigation of protein function and ligand binding capacity. Despite efforts to expand coverage of residues available for chemical proteomics (e.g., cysteine and lysine), a large fraction of the proteome remains inaccessible with current activity-based probes. Here, we introduce sulfur-triazole exchange (SuTEx) chemistry as a tunable platform for developing covalent probes with broad applications for chemical proteomics. We show modifications to the triazole leaving group can furnish sulfonyl probes with ~5-fold enhanced chemoselectivity for tyrosines over other nucleophilic amino acids to investigate more than 10,000 tyrosine sites in lysates and live cells. We discover that tyrosines with enhanced nucleophilicity are enriched in enzymatic, protein–protein interaction and nucleotide recognition domains. We apply SuTEx as a chemical phosphoproteomics strategy to monitor activation of phosphotyrosine sites. Collectively, we describe SuTEx as a biocompatible chemistry for chemical biology investigations of the human proteome. Sulfur-triazole exchange (SuTEx) chemistry is a tunable platform for covalent chemoproteomic probes that selectively target tyrosines, used to identify residues with enhanced nucleophilicity and monitor activation of phosphotyrosine sites.
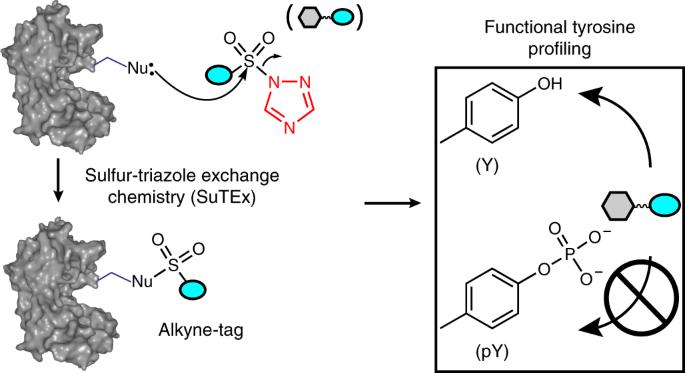
利用硫三唑交换化学研究功能性酪氨酸的全局靶向性
共价探针是全面研究蛋白质功能和配体结合能力的重要工具。尽管我们努力扩大可用于化学蛋白质组学的残基(如半胱氨酸和赖氨酸)的覆盖范围,但目前基于活性的探针仍有很大一部分蛋白质组无法访问。在这里,我们介绍了硫-三唑交换(SuTEx)化学,将其作为开发共价探针的可调平台,广泛应用于化学蛋白质组学。我们展示了对三唑离去基团的修饰可以提供磺酰基探针,与其他亲核氨基酸相比,磺酰基探针对酪氨酸的化学选择性提高了约 5 倍,可用于研究裂解物和活细胞中的 10,000 多个酪氨酸位点。我们发现,亲核性增强的酪氨酸富集于酶作用域、蛋白质-蛋白质相互作用域和核苷酸识别域。我们将 SuTEx 作为一种化学磷酸蛋白组学策略来监测磷酸酪氨酸位点的活化。总之,我们将 SuTEx 描述为一种用于人类蛋白质组化学生物学研究的生物兼容化学。硫-三唑交换(SuTEx)化学是一种可调节的共价化学蛋白质组探针平台,可选择性地靶向酪氨酸,用于鉴定亲核性增强的残基和监测磷酸酪氨酸位点的活化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


