Mechanically interlocked pyrene-based photocatalysts
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 9
Abstract
Triplet excited-state organic chromophores present countless opportunities for applications in photocatalysis. Here we describe an approach to the engineering of the triplet excited states of aromatic chromophores, which involves incorporating pyrene into pyridinium-containing mechanically interlocked molecules (MIMs). The π-extended nature of the pyrenes enforces [π···π] stacking, affording an efficient synthesis of tetrachromophoric octacationic homo[2]catenanes. These MIMs generate triplet populations and efficient intersystem crossing on account of the formation of a mixed charge-transfer/exciplex electronic state and a nanoconfinement effect, which leads to a high level of protection of the triplet state and extends the triplet lifetimes and yields. These compounds display excellent catalytic activity in photo-oxidation, as demonstrated by the aerobic oxidation of a sulfur-mustard simulant. This research highlights the benefits of using the mechanical bond to fine-tune the triplet photophysics of existing aromatic chromophores, providing an avenue for the development of unexplored MIM-based photosensitizers and photocatalysts. Although pyrene-containing molecules have been studied for their optical properties, the outcome of their incorporation into mechanically interlocked structures remains underexplored. Here, the authors install pyrene units into homo[2]catenanes and investigate the formation of long-lived triplet states, which can be exploited for photocatalysis.
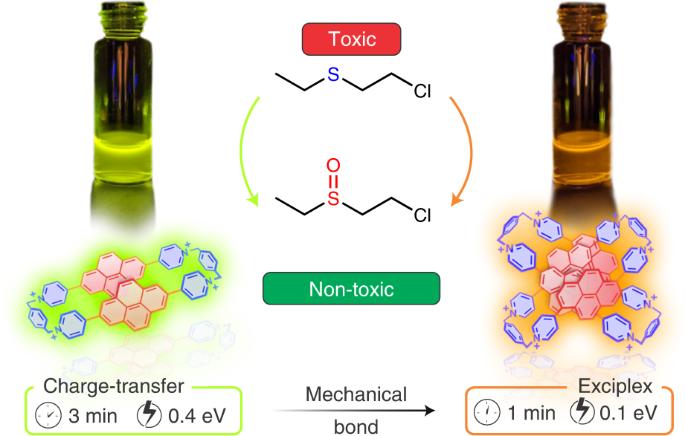
机械互锁芘基光催化剂
三重激发态有机发色团为光催化应用提供了无数机会。在这里,我们介绍了一种芳香族发色团三重激发态的工程学方法,即把芘加入到含吡啶的机械互锁分子(MIMs)中。芘的π-扩展性质强制了[π--π]堆叠,从而高效合成了四色八阳离子同[2]卡替烷。由于形成了混合电荷转移/外复电子态和纳米锎效应,这些 MIM 产生了三重态群和高效的系统间交叉,从而实现了对三重态的高度保护,并延长了三重态的寿命和产率。这些化合物在光氧化方面显示出卓越的催化活性,硫-芥末模拟物的有氧氧化就证明了这一点。这项研究强调了利用机械键微调现有芳香族发色团三重光物理学的好处,为开发基于 MIM 的未开发光敏剂和光催化剂提供了途径。尽管人们已经对含芘分子的光学特性进行了研究,但对其加入机械互锁结构的结果仍未进行深入探讨。在本文中,作者将芘单元安装到同[2]双烯烃中,并研究了长寿命三重态的形成,这种三重态可用于光催化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


