Targeting interleukin-13 receptor α2 (IL-13Rα2) for glioblastoma therapy with surface functionalized nanocarriers.
IF 6.5
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 2
Abstract
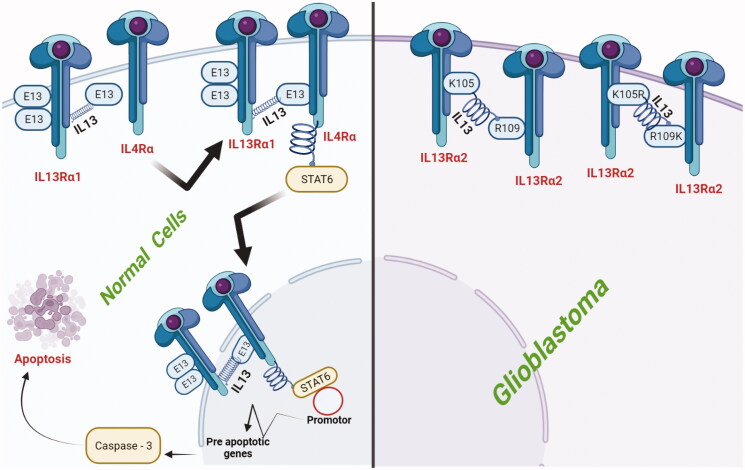
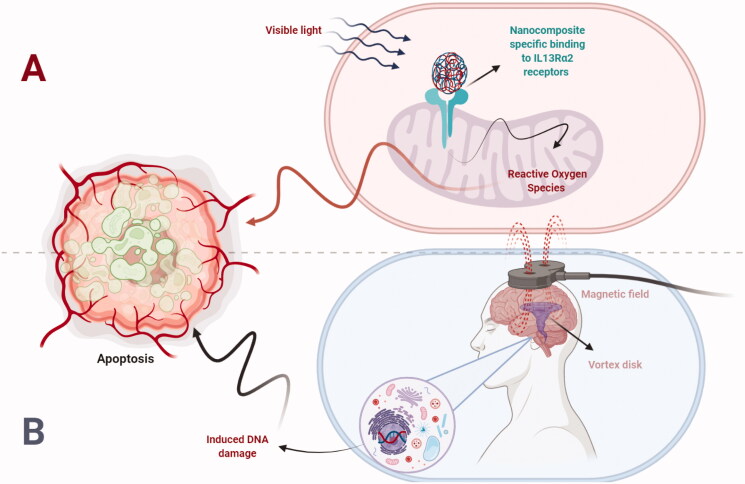
Abstract Despite surgical and therapeutic advances, glioblastoma multiforme (GBM) is among the most fatal primary brain tumor that is aggressive in nature. Patients with GBM have a median lifespan of just 15 months when treated with the current standard of therapy, which includes surgical resection and concomitant chemo-radiotherapy. In recent years, nanotechnology has shown considerable promise in treating a variety of illnesses, and certain nanomaterials have been proven to pass the blood–brain barrier (BBB) and stay in glioblastoma tissues. Recent preclinical research suggests that the diagnosis and treatment of brain tumor is significantly explored through the intervention of nanomaterials that has showed enhanced effect. In order to elicit an antitumor response, it is necessary to retain the therapeutic candidates within glioblastoma tissues and this job is effectively carried out by nanocarrier particularly functionalized nanocarriers. In the arena of neoplastic diseases including GBM have achieved great attention in recent decades. Furthermore, interleukin-13 receptor α chain variant 2 (IL13Rα2) is a highly expressed and studied target in GBM that is lacked by the surrounding environment. The absence of IL13Rα2 in surrounding normal tissues has made it a suitable target in glioblastoma therapy. In this review article, we highlighted the role of IL13Rα2 as a potential target in GBM along with design and fabrication of efficient targeting strategies for IL13Rα2 through surface functionalized nanocarriers.
靶向白细胞介素-13受体α2 (IL-13Rα2)的表面功能化纳米载体治疗胶质母细胞瘤
摘要尽管手术和治疗取得了进展,多形性胶质母细胞瘤(GBM)是最致命的原发性脑肿瘤之一,具有侵袭性。按照目前的治疗标准,GBM患者的中位寿命仅为15个月,其中包括手术切除和联合放化疗。近年来,纳米技术在治疗各种疾病方面显示出相当大的前景,某些纳米材料已被证明可以通过血脑屏障(BBB)并停留在胶质母细胞瘤组织中。最近的临床前研究表明,通过显示出增强效果的纳米材料的干预,对脑肿瘤的诊断和治疗进行了重大探索。为了引发抗肿瘤反应,有必要将候选治疗药物保留在胶质母细胞瘤组织中,而这项工作是通过纳米载体特别是功能化的纳米载体有效完成的。近几十年来,包括GBM在内的肿瘤性疾病引起了人们的极大关注。此外,白细胞介素13受体α链变体2(IL13Rα2)是GBM中一个高表达和研究的靶点,而周围环境缺乏该靶点。IL13Rα2在周围正常组织中的缺失使其成为胶质母细胞瘤治疗的合适靶点。在这篇综述文章中,我们强调了IL13Rα2作为GBM中潜在靶点的作用,以及通过表面功能化纳米载体设计和制造IL13Rβ2的有效靶向策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Drug Delivery
医学-药学
CiteScore
11.80
自引率
5.00%
发文量
250
审稿时长
3.3 months
期刊介绍:
Drug Delivery is an open access journal serving the academic and industrial communities with peer reviewed coverage of basic research, development, and application principles of drug delivery and targeting at molecular, cellular, and higher levels. Topics covered include all delivery systems including oral, pulmonary, nasal, parenteral and transdermal, and modes of entry such as controlled release systems; microcapsules, liposomes, vesicles, and macromolecular conjugates; antibody targeting; protein/peptide delivery; DNA, oligonucleotide and siRNA delivery. Papers on drug dosage forms and their optimization will not be considered unless they directly relate to the original drug delivery issues. Published articles present original research and critical reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


