In silico simulation of the effect of hypoxia on MCF-7 cell cycle kinetics under fractionated radiotherapy
Abstract
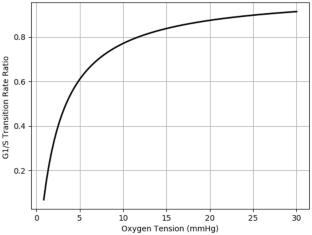
The treatment outcome of a given fractionated radiotherapy scheme is affected by oxygen tension and cell cycle kinetics of the tumor population. Numerous experimental studies have supported the variability of radiosensitivity with cell cycle phase. Oxygen modulates the radiosensitivity through hypoxia-inducible factor (HIF) stabilization and oxygen fixation hypothesis (OFH) mechanism. In this study, an existing mathematical model describing cell cycle kinetics was modified to include the oxygen-dependent G1/S transition rate and radiation inactivation rate. The radiation inactivation rate used was derived from the linear-quadratic (LQ) model with dependence on oxygen enhancement ratio (OER), while the oxygen-dependent correction for the G1/S phase transition was obtained from numerically solving the ODE system of cyclin D-HIF dynamics at different oxygen tensions. The corresponding cell cycle phase fractions of aerated MCF-7 tumor population, and the resulting growth curve obtained from numerically solving the developed mathematical model were found to be comparable to experimental data. Two breast radiotherapy fractionation schemes were investigated using the mathematical model. Results show that hypoxia causes the tumor to be more predominated by the tumor subpopulation in the G1 phase and decrease the fractional contribution of the more radioresistant tumor cells in the S phase. However, the advantage provided by hypoxia in terms of cell cycle phase distribution is largely offset by the radioresistance developed through OFH. The delayed proliferation caused by severe hypoxia slightly improves the radiotherapy efficacy compared to that with mild hypoxia for a high overall treatment duration as demonstrated in the 40-Gy fractionation scheme.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


