Lipopolysaccharide increases exosomes secretion from endothelial progenitor cells by toll-like receptor 4 dependent mechanism
Abstract
Background Information
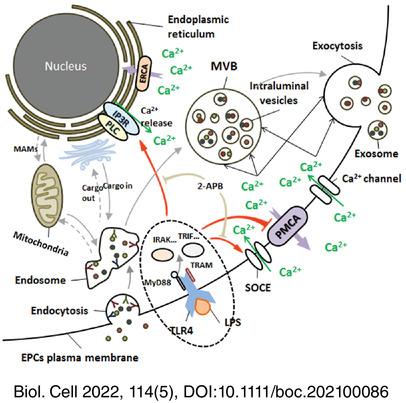
Endothelial progenitor cells (EPCs) can exert angiogenic effects by a paracrine mechanism, where exosomes work as an important mediator. Recent studies reported functional expression of toll-like receptor (TLR) 4 on human EPCs and dose-dependent effects of lipopolysaccharide (LPS) on EPC angiogenic properties. To study the effects of TLR4/LPS signaling on EPC-derived exosomes (Exo) and clarify the mechanism, we investigated the role of LPS on exosomes secretion from human EPCs and tested their anti-oxidation/senescence functions. We employed the inhibitors of the plasma membrane Ca2+-ATPase (PMCA), endoplasmic reticulum Ca2+-ATPase (ERCA), PLC-IP3 pathway and store-operated calcium entry to assess the effects of LPS on EPC intracellular calcium signalings which critical for exosome secretion.
Results
LPS induced the release of Exo in a TLR4-dependent manner in vitro, which effect can be partly abrogated by an membrane-permeable IP 3 R antagonist, 2-aminoethyl diphenylborinate (2-APB), but not PLC inhibitor, U-73122. The LPS can significantly delay the fallback of [Ca2+]i after isolating the cellular PMCA activity, and disturb PMCA 1/4 expression. The distribution of elevated intracellular calcium seemed coincident with the development of the multivesicular bodies (MVBs). furthermore, the anti-oxidation/senescence properties of LPS-induced Exo were validated by the senescence-associated β-galactosidase activity assay and reactive oxygen species (ROS) related H2DCF-DA assay.
Conclusions
The mechanism of PMCA downregulation and IP3R-dependent ER Ca2+ release may contribute to the pro-exosomal effects of LPS on EPCs.
Significance
This study provides new insights into the potential role of LPS/TLR4 pathway in regulating EPC-derived exosomes, which may help to develop some feasible approach to manipulate the Exo secretion and promote the clinical application of EPCs therapy in future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


