Nickel (II) Phenylthiosemicarbazone Complex Induces Cytotoxicity, Oxidative Stress and Apoptosis in Human Leukemia Stem-like KG1a Cells
Abstract
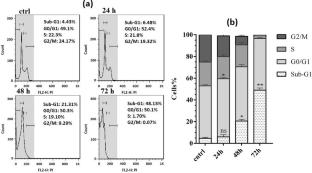
Thiosemicarbazones (TSCs) are compounds with different biological properties. Previous studies suggested that Thiosemicarbazones and their metal complexes have potential cytotoxic effects against numerous cancers. In the present study, the anti-proliferative effect and oxidative stress-induced apoptosis in the human leukemia stem-like KG1a cells by Nickel (II) phenylthiosemicarbazone complex (4-NPTC) were evaluated. For this purpose, the KG1a cells were treated with different concentrations of the 4-NPTC to determine the IC50 value by MTT assay. We investigated the impact of 4-NPTC on induction of oxidative stress by measuring the activity of two antioxidant enzymes, catalase (CAT) and superoxide dismutase (SOD), and by assessing the level of biomarkers such as malondialdehyde (MDA), total thiol and ROS levels in the leukemia stem-like KG1a cells. Moreover, we evaluated the influence of 4-NPTC on the cell cycle progression using flow cytometry. Results showed that 4-NPTC enhanced the activity of antioxidant enzymes in the cells after 24 h exposure and decreased their activity when the exposure period to 4-NPTC was prolonged to 48 h and 72 h. Furthermore, the notably enhance of intracellular ROS levels after 48 and 72 h was observed. In addition, the diminution of intracellular thiol enhanced in MDA amounts was observed in treated KG1a cells. The G0/G1 phase cell cycle arrest followed by induction of apoptosis was shown in KG1a cells after treatment with the 4-NPTC. Together, the results demonstrate that 4-NPTC plays important role in blocking of development of the leukemic cells by inducing oxidative stress and apoptosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


