Atomic metal–non-metal catalytic pair drives efficient hydrogen oxidation catalysis in fuel cells
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
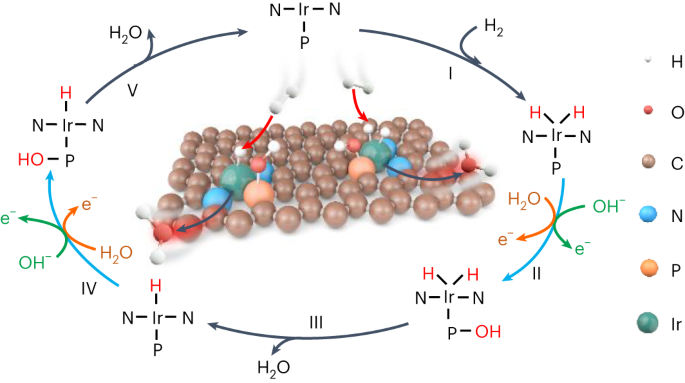
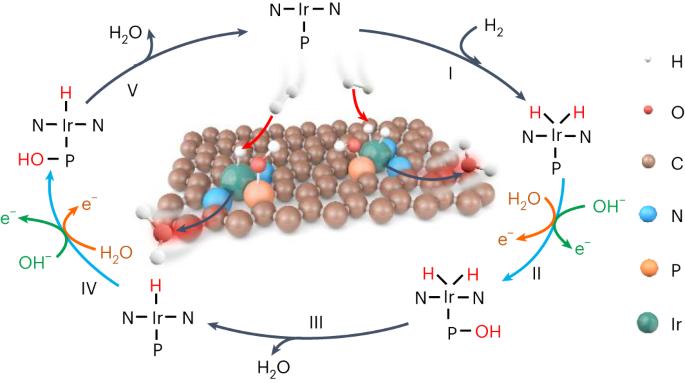
Rational design of efficient hydrogen oxidation reaction (HOR) electrocatalysts with maximum utilization of platinum-group metal sites is critical to hydrogen fuel cells, but remains a major challenge due to the formidable potential-dependent energy barrier for hydrogen intermediate (H*) desorption on single metal centres. Here we report atomically dispersed iridium–phosphorus (Ir–P) catalytic pairs with strong electronic coupling that integratively facilitate HOR kinetics, in which the reactive hydroxyl species adsorbed on the more oxophilic P site induces an alternative thermodynamic pathway to facilely combine with H* on the adjacent Ir atom, whereas isolated single-atom Ir catalysts are inactive. In H2–O2 fuel cells, this catalyst enables a peak power density of 1.93 W cm−2 and an anodic mass activity as high as 17.11 A mgIr−1 at 0.9 ViR-free, significantly outperforming commercial Pt/C. This work not only advances the development of anodic catalysts for fuel cells, but also provides a precise and universal active-site design principle for multi-intermediate catalysis. Fuel cells rely on costly and scarce platinum-group metals to catalyse both anodic and cathodic reactions. Here a catalyst consisting of atomically dispersed iridium and phosphorus on carbon is presented, where adjacent iridium and phosphorus sites work as integrative catalytic pairs to synergically boost the performance for the hydrogen oxidation reaction.
金属-非金属原子催化对驱动燃料电池中高效的氢氧化催化
合理设计可最大限度利用铂族金属位点的高效氢氧化反应(HOR)电催化剂对氢燃料电池至关重要,但由于氢中间体(H*)在单个金属中心上的解吸存在巨大的电位能障,因此这仍是一项重大挑战。在这里,我们报告了原子分散的铱-磷(Ir-P)催化对,它们具有很强的电子耦合,能综合促进氢中间体动力学,其中吸附在亲氧化性更强的 P 位点上的活性羟基物种能诱导另一种热力学途径,使其与相邻 Ir 原子上的 H* 便捷结合,而孤立的单原子 Ir 催化剂则没有活性。在 H2-O2 燃料电池中,这种催化剂的峰值功率密度为 1.93 W cm-2,在 0.9 ViR-free 条件下,阳极质量活性高达 17.11 A mgIr-1,明显优于商用 Pt/C。这项工作不仅推动了燃料电池阳极催化剂的发展,还为多中间体催化提供了精确、通用的活性位设计原理。燃料电池依靠昂贵而稀缺的铂族金属来催化阳极和阴极反应。这里介绍的是一种由碳上原子分散的铱和磷组成的催化剂,其中相邻的铱和磷位点作为整合催化对协同提高氢氧化反应的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


