Promoting Cu-catalysed CO2 electroreduction to multicarbon products by tuning the activity of H2O
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The electrochemical reduction of CO2 to valuable C2+ feedstocks is hindered by the competitive formation of C1 products and H2 evolution. Here we tuned the H2O thermodynamic activity between 0.97 and 0.47 using water-in-salt electrolytes to obtain mechanistic insights into the role of H2O in controlling C–C coupling versus C1 product formation on Cu electrodes. By lowering the thermodynamic H2O activity to 0.66, we obtained a Faradaic efficiency of ~73% at a partial current density of −110 mA cm−2 for C2+ products, at modest overpotentials. The adjustment of the thermodynamic H2O activity provided fine control over C2+/C1 ratios, spanning a range from 1 to 20. The trends support the pivotal role of the thermodynamic H2O activity in increasing the CO surface coverages and promoting C–C coupling to C2 products. These findings highlight the potential of tuning thermodynamic H2O activity as a guiding principle to maximize CO2 reduction into highly desirable C2+ products. Copper-based electrocatalysts promote the formation of high-value multicarbon products from CO2, but the process competes with C1 product formation. Now a strategy is presented to tune the activity of water by using water-in-salt electrolytes to increase the C2+/C1 ratio.
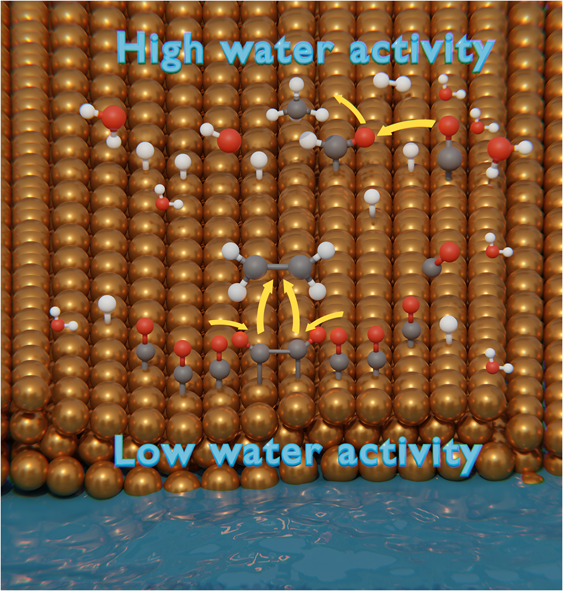
通过调节水的活性促进cu催化CO2电还原成多碳产物
将二氧化碳电化学还原成有价值的 C2+ 原料的过程受到 C1 产物的竞争性形成和 H2 演化的阻碍。在此,我们利用盐包水型电解质在 0.97 和 0.47 之间调整了 H2O 的热力学活性,以便从机理上深入了解 H2O 在控制铜电极上 C-C 偶联与 C1 产物形成中的作用。通过将热力学 H2O 活性降低到 0.66,我们在部分电流密度为 -110 mA cm-2 时获得了约 73% 的法拉第效率,C2+ 产物的过电位适中。热力学 H2O 活性的调整提供了对 C2+/C1 比率的精细控制,范围从 1 到 20。这些趋势证明了热力学 H2O 活性在增加 CO 表面覆盖率和促进 C-C 偶联生成 C2 产物方面的关键作用。这些发现凸显了调整热力学 H2O 活性作为指导原则的潜力,可最大限度地将 CO2 还原成非常理想的 C2+ 产物。铜基电催化剂能促进 CO2 生成高价值的多碳产品,但这一过程与 C1 产品的生成存在竞争。现在介绍一种策略,通过使用盐中水电解质来调节水的活性,从而提高 C2+/C1 的比例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


