Comparison of polyampholyte derivative of chitosan with bisphthalimides of low molecular weight in the green synthesis of Au nanoparticles
Abstract
Abstract
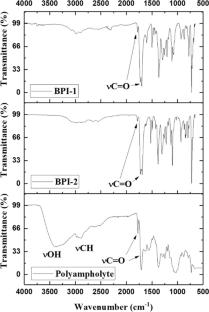
This work reports the chemical modification of chitosan with phthalimide moieties and the synthesis of two bisphthalimides (BPIs) with low molecular weight useful in the green synthesis of Au nanoparticles. The polyampholyte derivative of chitosan was obtained in water while the two BPIs, N,N´-(1,3-phenylene)bis(phthalimide-5-carboxylic acid) (BPI-1) and N,N´-(1,4-phenylene)bis(phthalimide-5-carboxylic acid) (BPI-2) were obtained by solid state reaction without the use of organic solvents. The polyampholyte and two BPIs were soluble in water; therefore, they were used in the green synthesis of Au nanoparticles. The characterization of three compounds was made by FTIR and 1H NMR. TEM and UV–Vis results revealed that the compounds can reduce gold ions and stabilize Au nanoparticles in the colloidal solutions. The shape of the Au nanoparticles was quasi-spherical, while the average size of Au nanoparticles stabilized with the compounds BPI-1 and BPI-2 was 3 and 12 nm, respectively. Thus, with BPI-1 was obtained Au nanoparticles with lower size than BPI-2. Finally, the average size of nanoparticles stabilized with the polyampholyte was 10 nm. However, the faster reaction to obtain nanoparticles was with the polyampholyte derivative of chitosan (5 min), due to high quantity of oxidizable groups found in the polymer structure by its high molecular weight.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


