Na+,K+-ATPase As a Polyfunctional Protein
Abstract—
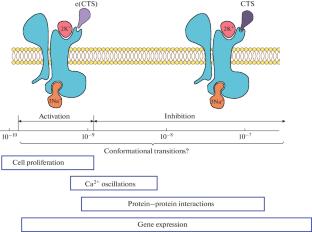
Since the discovery of Na+,K+-ATPase by Jens Skou in 1957, this enzyme has been considered exclusively as a transporter that ensures the active transport of Na+ and K+ ions across the cell plasma membrane; therefore, its structure and mechanism of functioning, as well as its involvement in secondary ion transport systems have been studied in detail. In the present review, the data on the structure and functioning of the enzyme are briefly reviewed. The role of Na+,K+-ATPase as a receptor for cardiotonic steroids (CTS), whose binding to the enzyme initiates a variety of signaling pathways through protein–protein interactions modified also by changes in the intracellular concentration of Na+ and K+ ions by inhibiting the Na+,K+-ATPase transport function and Ca2+, by mediating changes in Na/Ca-exchange activity, was described in more detail. All these provide a variety of CTS effects, including their effect on gene expression, the state of tight junctions, cell adhesion, induction of myocardial hypertrophy, stimulation of free-radical oxygen species generation, and initiation of cell death or survival depending on tissue type. Data on the discovery of endogenous CTS are presented, as well as an analysis of published data indicating that concentrations of endogenous CTS are so low that they are unlikely to cause inhibition of Na+,K+-ATPase. In this connection, the data on the enzyme activation by low doses of CTS are presented, and the idea of a possible summation of the concentrations of various steroids is suggested. Possible directions for the study of multiple functions of Na+,K+-ATPase are discussed in the conclusion.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


