Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 25
Abstract
The chemistry of carbohydrates has a history of over 100 years, but simple, stereoselective and efficient glycosylation methods remain highly needed to facilitate the studies of sugars in various disciplines. Here we report a strategy for 1,2-cis-glycosylation without using metals, strong (Lewis) acids, elaborate catalysts or labile substrates. Our method operates by a unique mechanism: it activates glycosyl donors through a radical cascade rather than the conventional acid-promoted, ionic process. As elucidated by computational and experimental studies, the allyl glycosyl sulfones (as donors) form halogen bond complexes with perfluoroalkyl iodides, which—merely by visible light irradiation—fragment via radical intermediates to give the electrophilic glycosyl iodides. In situ trapping by various nucleophiles affords, in a stereoconvergent manner, the challenging 1,2-cis-glycosides. This metal- and acid-free reaction shows remarkable tolerance to functional groups. The high stereoselectivity holds for a broad array of donors. This study suggests that the simple C2-alkoxy group can serve as an effective directing group for building 1,2-cis-glycosidic bonds. Most chemical glycosylation methods operate by acid-promoted, ionic activation of donors. Now, by exploiting the formation of a halogen-bond complex, the activation of glycosyl donors was achieved via a visible light-promoted radical cascade process, resulting in a general, simple and mild way to build challenging 1,2-cis-glycosidic bonds.
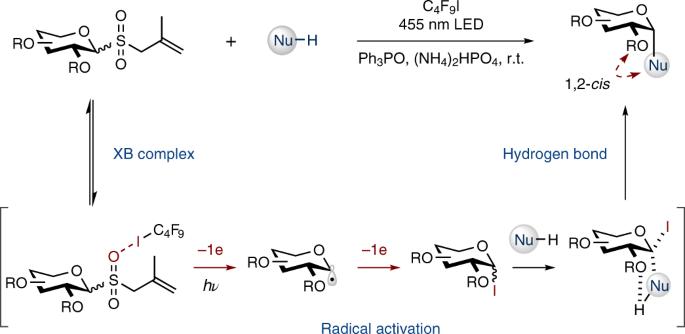
糖基供体的卤素键辅助自由基活化使1,2-顺式糖基化具有温和和立体收敛性
碳水化合物化学已有 100 多年的历史,但要促进各学科对糖类的研究,仍然非常需要简单、立体选择性和高效的糖基化方法。在这里,我们报告了一种不使用金属、强(路易斯)酸、复杂催化剂或易变底物的 1,2-顺式糖基化策略。我们的方法以一种独特的机制运行:它通过自由基级联激活糖基供体,而不是传统的酸促进离子过程。计算和实验研究表明,烯丙基糖基砜(作为供体)与全氟烷基碘化物形成卤键络合物,只需用可见光照射,这些络合物就会通过自由基中间体发生分裂,生成亲电的糖基碘化物。通过各种亲核物的原位捕获,以立体转换的方式得到具有挑战性的 1,2-顺式糖苷。这种不含金属和酸的反应对官能团具有显著的耐受性。这种高立体选择性适用于多种供体。这项研究表明,简单的 C2-烷氧基可以作为构建 1,2-顺式糖苷键的有效指导基团。大多数化学糖基化方法都是通过酸促进供体的离子活化来实现的。现在,利用卤素键复合物的形成,通过可见光促进的自由基级联过程实现了糖基供体的活化,从而以一种通用、简单而温和的方法建立了具有挑战性的 1,2-顺式糖苷键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


