In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 154
Abstract
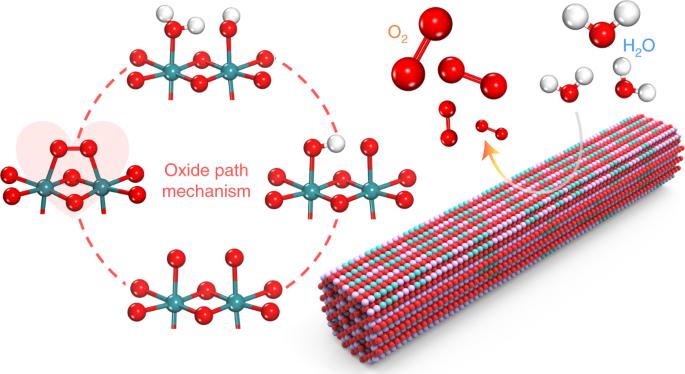
The development of acid-stable oxygen evolution reaction electrocatalysts is essential for high-performance water splitting. Here, we report an electrocatalyst with Ru-atom-array patches supported on α-MnO2 (Ru/MnO2) for the oxygen evolution reaction following a mechanism that involves only *O and *OH species as intermediates. This mechanism allows direct O–O radical coupling for O2 evolution. Ru/MnO2 shows high activity (161 mV at 10 mA cm−2) and outstanding stability with small degradation after 200 h operation, making it one of the best-performing acid-stable oxygen evolution reaction catalysts. Operando vibrational and mass spectroscopy measurements were performed to probe the reaction intermediates and gaseous products for validating the oxygen evolution reaction pathway. First-principles calculations confirmed the cooperative catalysis mechanism with a reduced energy barrier. Time-dependent elemental analysis demonstrated the occurrence of the in-situ dynamic cation exchange reaction during the oxygen evolution reaction, which is the key for triggering the reconstruction of Ru atoms into the ordered array with high durability. Proton exchange membrane water electrolysers require the development of active, stable and cost-effective catalysts for water oxidation. Now, a Ru/α-MnO2 catalyst with in-situ-formed arrays of Ru atoms is presented for acidic water oxidation, which follows the oxide path mechanism and achieves enhanced activity and stability.
在α-MnO2上原位重建Ru原子阵列,提高了酸性水氧化性能
开发酸性稳定的氧进化反应电催化剂对高性能水分离至关重要。在此,我们报告了一种在 α-MnO2(Ru/MnO2)上支撑 Ru 原子阵列贴片的电催化剂,其氧进化反应的机理只涉及 *O 和 *OH 物种作为中间体。这种机理允许 O-O 自由基直接耦合进行 O2 演化。Ru/MnO2 表现出很高的活性(10 mA cm-2 时为 161 mV)和出色的稳定性,运行 200 小时后降解很小,使其成为性能最好的酸稳定性氧进化反应催化剂之一。为了验证氧进化反应途径,对反应中间产物和气态产物进行了操作性振动和质谱测量。第一原理计算证实了能障降低的合作催化机制。随时间变化的元素分析表明,在氧进化反应过程中发生了原位动态阳离子交换反应,这是引发 Ru 原子重构为高耐久性有序阵列的关键。质子交换膜水电解槽需要开发活性、稳定和经济高效的水氧化催化剂。现在,我们提出了一种具有原位形成的 Ru 原子阵列的 Ru/α-MnO2 催化剂,用于酸性水的氧化,该催化剂遵循氧化物路径机制,具有更高的活性和稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


