Complex of antamanide with the nitrate anion
IF 2.3
4区 化学
Q2 Agricultural and Biological Sciences
Journal of Inclusion Phenomena and Macrocyclic Chemistry
Pub Date : 2023-08-16
DOI:10.1007/s10847-023-01199-w
引用次数: 0
Abstract
Anionic complex of antamanide with the nitrate anion has been proven by electrospray ionization mass spectrometry (ESI-MS) method. Further, applying quantum chemical DFT calculations, the most probable structure of this complex was derived. The nitrate anion is embedded in the molecule of antamanide and its oxygens atoms are bonded by seven bonds to the hydrogen atoms of the ligand. Finally, the interaction energy, E(int), of the antamanide-NO3− complex was calculated as E(int) = -175.9 kJ/mol.
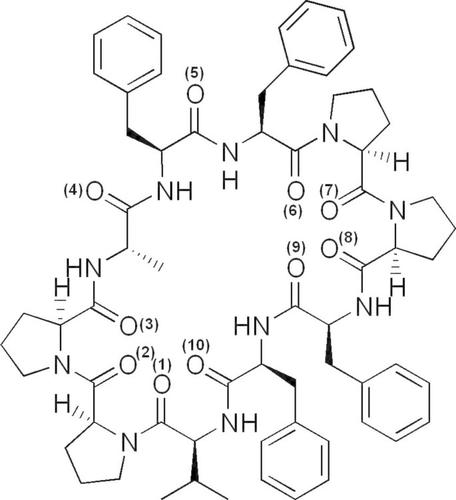
安他胺与硝酸根阴离子的配合物
采用电喷雾质谱法(ESI-MS)证实了氨他胺与硝酸根阴离子的阴离子配合物。进一步,应用量子化学DFT计算,推导出该配合物最可能的结构。硝酸根阴离子嵌在胺酰胺分子中,它的氧原子通过7个键与配体的氢原子相连。最后计算出氨酰胺- no3−配合物的相互作用能E(int)为E(int) = -175.9 kJ/mol。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.30
自引率
8.70%
发文量
0
审稿时长
3-8 weeks
期刊介绍:
The Journal of Inclusion Phenomena and Macrocyclic Chemistry is the premier interdisciplinary publication reporting on original research into all aspects of host-guest systems. Examples of specific areas of interest are: the preparation and characterization of new hosts and new host-guest systems, especially those involving macrocyclic ligands; crystallographic, spectroscopic, thermodynamic and theoretical studies; applications in chromatography and inclusion polymerization; enzyme modelling; molecular recognition and catalysis by inclusion compounds; intercalates in biological and non-biological systems, cyclodextrin complexes and their applications in the agriculture, flavoring, food and pharmaceutical industries; synthesis, characterization and applications of zeolites.
The journal publishes primarily reports of original research and preliminary communications, provided the latter represent a significant advance in the understanding of inclusion science. Critical reviews dealing with recent advances in the field are a periodic feature of the journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


