A rational preparation strategy of phase tuned MoO3 nanostructures for high-performance all-solid asymmetric supercapacitor
Abstract
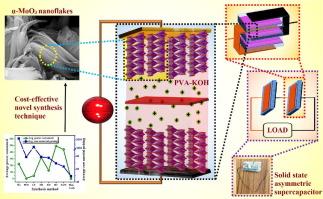
In this work, phase and morphology-tuned MoO3 nanostructures are synthesized through a novel modified co-precipitation method, and their electrochemical properties are investigated. For the first time, such a simple surfactant-assisted synthesis process aided by minor temperature variations is reported which results in phase transition of the nanoparticles from h-MoO3 nano-rods to α-MoO3 nano-flakes. The nanostructures thus developed are highly porous and crystalline with significantly large specific surface area as compared to previous literature. The theoretical bandgap energy of the optimized sample calculated using Perdew-Zunger local density approximation (LDA) is in good agreement with the experimental findings. An overall structural, morphological, and surface-behavioural analysis predicts the electrochemical superiority in 2D α-MoO3. The cyclic voltammetry and galvano-potentiometry measurements of 2D α-MoO3 in the potential window of −0.6 V to +0.2 V present the highest pseudo-supercapacitive response with a maximum specific capacitance of 829 F g−1 at 2 A g−1 as compared to h-MoO3 (452 F g−1) and h@α-MoO3 (783 F g−1). Thus, the MoO3 2D nanostructures synthesized through our novel synthesis technique display excellent specific capacitance as compared to previous reported data. Additionally, α-MoO3 exhibits a galvanostatic charging-discharging cyclic stability of about 91% after 2000 cycles, indicating that it can serve as an excellent electrode material for supercapacitors. A solid-state asymmetric supercapacitor device is successfully constructed using α-MoO3 which can light up 4 red LEDs for 10 s. The specific energy density of the device reaches a maximum value of 36.3 W h kg−1 at the power density of 50 W kg−1.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


