Operando visualization of the hydrogen evolution reaction with atomic-scale precision at different metal–graphene interfaces
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 54
Abstract
The development of catalysts for the hydrogen evolution reaction is pivotal for the hydrogen economy. Thin iron films covered with monolayer graphene exhibit outstanding catalytic activity, surpassing even that of platinum, as demonstrated by a method based on evaluating the noise in the tunnelling current of electrochemical scanning tunnelling microscopy. Using this approach, we mapped with atomic-scale precision the electrochemical activity of the graphene–iron interface, and determined that single iron atoms trapped within carbon vacancies and curved graphene areas on step edges are exceptionally active. Density functional theory calculations confirmed the sequence of activity obtained experimentally. This work exemplifies the potential of electrochemical scanning tunnelling microscopy as the only technique able to determine both the atomic structure and relative catalytic performance of atomically well-defined sites in electrochemical operando conditions and provides a detailed rationale for the design of novel catalysts based on cheap and abundant metals such as iron. Establishing structure–activity relationships is crucial for the design of improved catalysts. Now, by developing a method based on electrochemical scanning tunnelling microscopy, the active sites of graphene/iron/platinum interfaces are visualized with atomic-scale precision in real time during the hydrogen evolution reaction.
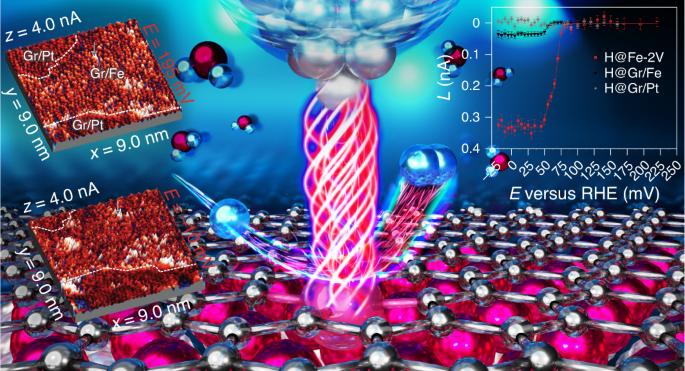
不同金属-石墨烯界面上原子级精度析氢反应的操作可视化
氢气进化反应催化剂的开发对氢经济至关重要。覆盖着单层石墨烯的薄铁薄膜表现出了卓越的催化活性,甚至超过了铂,这一点通过一种基于评估电化学扫描隧道显微镜隧道电流噪声的方法得到了证明。利用这种方法,我们以原子尺度精确绘制了石墨烯-铁界面的电化学活性图,并确定了被困在碳空位和阶梯边缘石墨烯弯曲区域内的单个铁原子异常活跃。密度泛函理论计算证实了实验所获得的活性序列。这项工作充分体现了电化学扫描隧道显微镜的潜力,它是唯一能够在电化学操作条件下确定原子结构和原子定义明确的位点的相对催化性能的技术,并为基于铁等廉价而丰富的金属设计新型催化剂提供了详细的理论依据。建立结构-活性关系对于设计改良催化剂至关重要。现在,通过开发一种基于电化学扫描隧道显微镜的方法,可以在氢进化反应过程中以原子尺度的精度实时观察石墨烯/铁/铂界面的活性位点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


