Regioisomers of 2,5,6,7,8-Pentaaryl-1H-Azepino[3,2,1-ij]Quinazoline-1,3(2H)-Dione Containing Various Aryl Substituents in the Azepine Ring: Structure Determination Using NMR Methods
Abstract
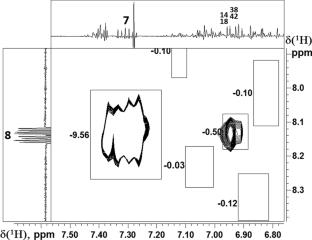
NMR spectroscopy methods were used to prove the structures of two similar regioisomers of 2,5,6,7,8-pentaaryl-1H-azepino[3,2,1-ij]quinazoline-1,3(2H)-dione containing various aryl substituents in the azepine ring which were obtained as reaction products and existed in CDCl3 as inseparable mixture of two compounds with almost equal (56:44) relation between them. Complete signal assignment in 1H and 13C spectra of each compound was made by using some homo- and heteronuclear NMR experiments. Long-range distance estimation (up to 5.0 Å) on the base of nuclear Overhauser enhancement approach (NOE) at conditions of extreme-narrow limits (ωoτc < < 1) was used to determine the quantitative level the internuclear distances between protons H6 and H8 situated in the rigid part of molecules and the nearest ortho- and meta-protons in mobile phenyl rings Ph5 and Ph2, respectively. The distance difference between the calculated and experimental values in all cases was not more than 10%. These results allowed us to prove that a dominant regioisomer (3a) has para-methoxy-substituted rings at positions 9 and 12 of seven-membered ring C, and a minor regioisomer (3d) has these rings at positions 10 and 12. The results of an independent approach based on the comparison of the chemical shifts of the 1H and 13C nuclei of the regioisomers under study are in full agreement (or do not contradict) with the obtained conclusions based on the quantitative NOE measurements of interproton distances. The methodological approach on the basis of long-range distance estimation by NOE tested in this work can be used to establish the structure of inseparable mixtures of two or more compounds or to solve similar problems under conditions of complex mixtures of closely related organic compounds.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


