Electrophilic cyclization of reticuline-type alkaloids in flow via o-quinol intermediates
IF 2
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
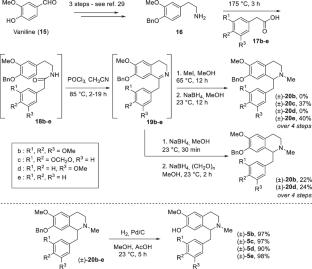
Herein, we report the first continuous-flow biomimetic cyclization of reticuline derivatives to aporphine alkaloids via ortho-quinol intermediates. The two-step flow process involves an initial oxidative dearomatization of reticuline derivatives to using hypervalent iodine(III) reagents, followed by a TMSOTf-mediated electrophilic cyclization. The high sensitivity of ortho-quinol compounds is mitigated by the mild experimental conditions and fast reaction rates offered by flow reactors. A preliminary structure–reactivity relationship suggests that both steps of the process are favored with strongly electron-rich substrates, similar to what is observed in nature.
网状型生物碱通过邻喹啉中间体的亲电环化
在这里,我们报道了第一个网状衍生物通过邻喹啉中间体连续流动的仿生环化到阿啡生物碱。两步流动过程包括网状衍生物的初始氧化去芳构化,使用高价碘(III)试剂,然后是tmsotf介导的亲电环化。流动反应器提供的温和的实验条件和快速的反应速率减轻了对邻喹啉类化合物的高灵敏度。初步的结构-反应性关系表明,该过程的两个步骤都有利于强富电子衬底,类似于在自然界中观察到的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Flow Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
6.40
自引率
3.70%
发文量
29
审稿时长
>12 weeks
期刊介绍:
The main focus of the journal is flow chemistry in inorganic, organic, analytical and process chemistry in the academic research as well as in applied research and development in the pharmaceutical, agrochemical, fine-chemical, petro- chemical, fragrance industry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


